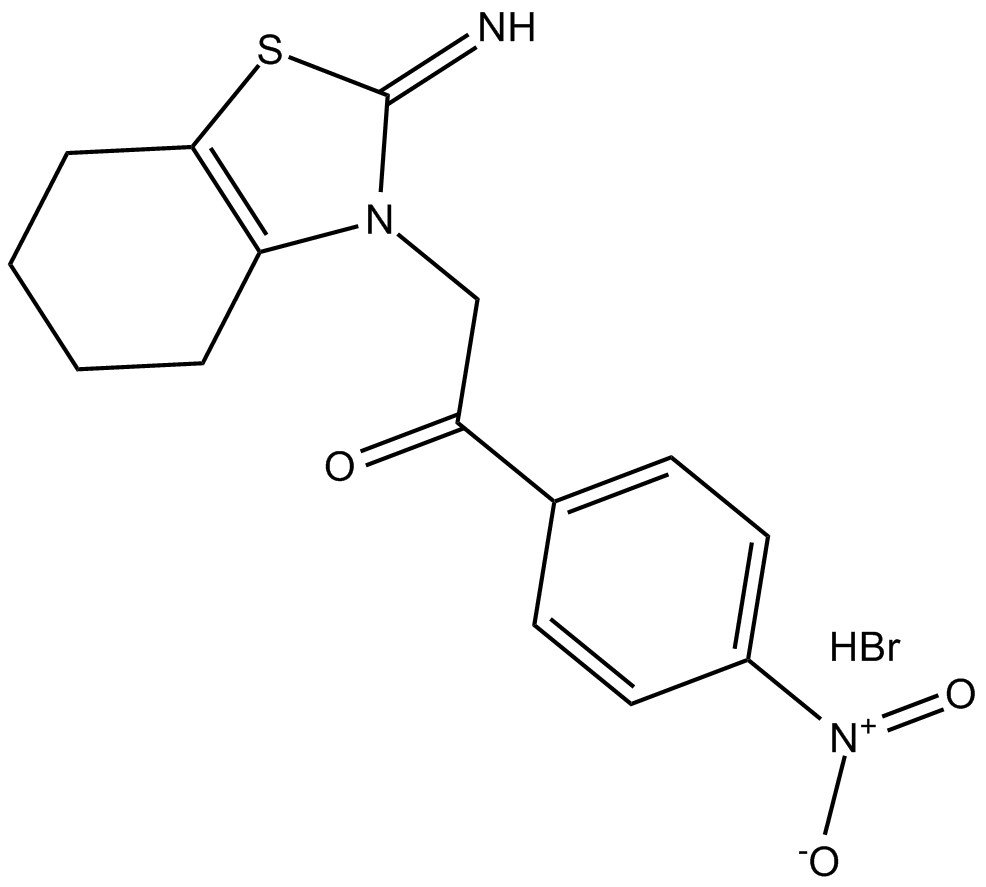
p-nitro-Pifithrin-α
p-nitro-Pifithrin-α, a cell-permeable cyclic analog of pifithrin-α, is an inhibitor of p53 activity [1].
The p53 tumor suppressor gene product can induce apoptotic cell death and plays a dominant role in apoptosis, genomic stability, and inhibition of angiogenesis. The p53 has been considered to be an oncogene and the wild-type gene product actually functions as a tumour suppressor gene. p53 mutations play an important role in the development of many common human malignancies [2].
In Vitro: In p53-/- cortical neuron, p-nitro-Pifithrin-α exihibited a p53 inhibitory activity in preventing p53-induced death[1]. p-nitro-Pifithrin-α did not prevent cortical neuronal death induced by p40Met, showing the remarkable specificity in the inhibitory action of p-nitro-Pifithrin-α on p53. p-nitro-Pifithrin-α (300 nM) prevented p53-triggered increase in protein levels of p21/WAF1, indicating that p-nitro-Pifithrin-α behaved as p53 posttranscriptional activity inhibitors. p-nitro-Pifithrin-α at a dose of 30 nM was sufficient to prevent the increase of p21/WAF1 levels [1]. p-nitro-Pifithrin-α was slowly converted into a more potent cyclized form, p-nitro cyclic pifithrin-α, when incubated in biological media (t1/2= 8 h)
In human proximal tubular cells, p-nitro-Pifithrin-α (10 μM) suppressed p53-mediated TGF-β1 expression [3].
In vivo: In a mouse model of non-alcoholic fatty liver disease, p-nitro-Pifithrin-α attenuated steatosis and liver injury in mice fed a high-fat diet [4].
References:
[1] Pietrancosta N, Moumen A, Dono R, et al. Imino-tetrahydro-benzothiazole derivatives as p53 inhibitors: discovery of a highly potent in vivo inhibitor and its action mechanism[J]. Journal of medicinal chemistry, 2006, 49(12): 3645-3652.
[2] Nigro J M, Baker S J, Preisinger A C, et al. Mutations in the p53 gene occur in diverse human tumour types[J]. Nature, 1989, 342(6250): 705-708.
[3] Shimizu H, Yisireyili M, Nishijima F, et al. Indoxyl sulfate enhances p53-TGF-β1-Smad3 pathway in proximal tubular cells[J]. American journal of nephrology, 2013, 37(2): 97-103.
[4] Derdak Z, Villegas K A, Harb R, et al. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease[J]. Journal of hepatology, 2013, 58(4): 785-791.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 398.3 |
| Cas No. | 389850-21-9 |
| Formula | C15H15N3O3S·HBr |
| Synonyms | p-nitro-PFT-α |
| Solubility | ≤1mg/ml in DMSO;1mg/ml in dimethyl formamide |
| Chemical Name | 1-(4-nitrophenyl)-2-(4,5,6,7-tetrahydro-2-imino-3(2H)-benzothiazolyl)-ethanone, monohydrobromide |
| SDF | Download SDF |
| Canonical SMILES | N=C1SC(CCCC2)=C2N1CC(c(cc1)ccc1[N+]([O-])=O)=O.Br |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity ≥95.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
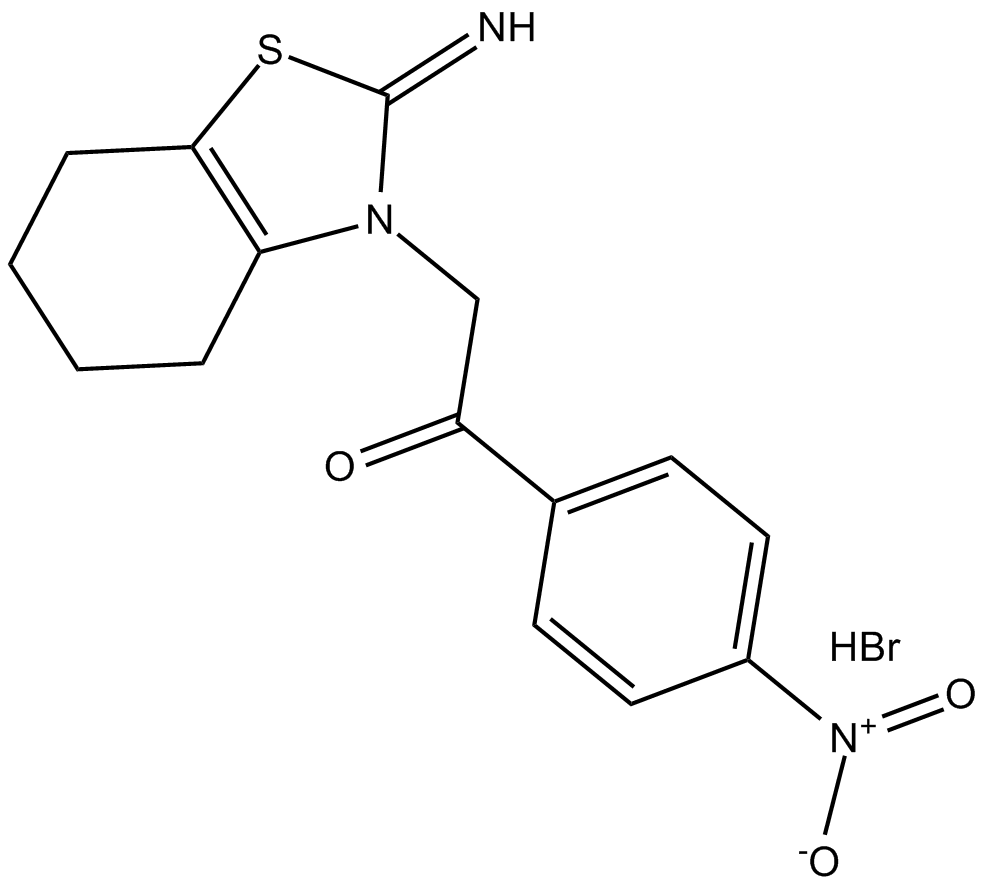
Chemical structure
Related Biological Data