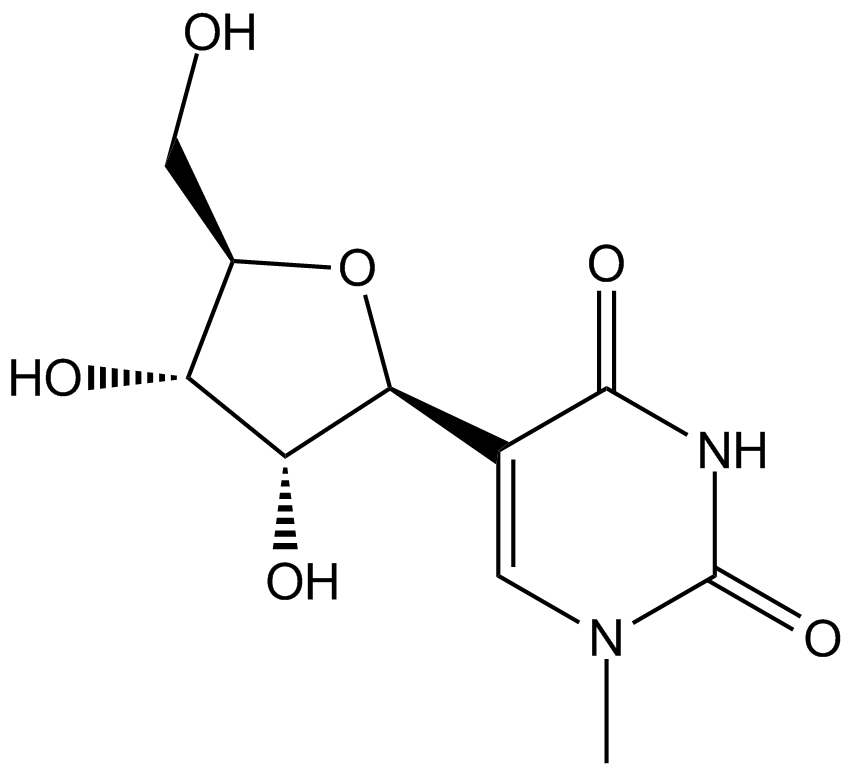
N1-Methylpseudouridine
N1-Methylpseudouridine is a modified nucleoside used for enhancing mRNA translation. Incorporation of N1-Methylpseudouridine into mRNA turns off the immune/eIF2α phosphorylation-dependent inhibition of translation, and increases ribosome pausing and density on the mRNA, thus dramatically facilitating the translation process. N1-Methylpseudouridine outperforms several other modified nucleosides (e.g. 5-Methylcytidine) and corresponding combinations in terms of translation capacity.
References:
1. Svitkin YV, Cheng YM, Chakraborty T, et al. N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Research, 2017, 45(10): 6023-6036.
2. Andries O, Mc Cafferty S, De Smedt SC, et al N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. Journal of Controlled Release, 2015, 217: 337-344.
- 1. Wang Jiahui, Yu Xiang, et al. "The mitochondrial DNAJC co-chaperone TCAIM reduces α-ketoglutarate dehydrogenase protein levels to regulate metabolism." Molecular Cell Volume 85, Issue 3P638-651.E9, February 06, 2025
- 2. Peilu She, Bangjun Gao, et al. "The transcriptional repressor HEY2 regulates mitochondrial oxidative respiration to maintain cardiac homeostasis." Nat Commun. 2025 Jan 2;16(1):232. PMID: 39747914
- 3. Jingjing Zhang, Yun Li, et al. "Genome-wide CRISPR/Cas9 library screen identifies PCMT1 as a critical driver of ovarian cancer metastasis." J Exp Clin Cancer Res. 2022 Jan 15;41(1):24. PMID: 35033172
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 258.23 |
| Cas No. | 13860-38-3 |
| Formula | C10H14N2O6 |
| Solubility | ≥50 mg/mL in H2O with ultrasonic; ≥20 mg/mL in EtOH; ≥20.65 mg/mL in DMSO |
| Chemical Name | 5-((2S,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1-methylpyrimidine-2,4(1H,3H)-dione |
| SDF | Download SDF |
| Canonical SMILES | CN(C=C([C@@H]([C@@H]1O)O[C@H](CO)[C@H]1O)C(N1)=O)C1=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[2] | |
|
Cell lines |
Various mammalian cells including A549, BJ, C2C12, HeLa and primary keratinocytes |
|
Reaction Conditions |
24 h incubation |
|
Applications |
In various mammalian cells, mRNAs in the N1-Methylpseudouridine + 5-Methylcytidine group showed reduced cytotoxicity compared to the Pseudouridine + 5-Methylcytidine group. The mRNAs simultaneously modified by N1-Methylpseudouridine and 5-Methylcytidine also exhibited reduced activation of the intracellular innate immune response. |
| Animal experiment:[2] | |
|
Animal models |
7-week-old Balb/c mice |
|
Dosage form |
20 μg Once daily through intradermal or intramuscular routes by lipofection, for 21 days |
|
Applications |
N1-Methylpseudouridine alone and its combination with 5-Methylcytidine outperformed the current state-of-the-art Pseudouridine and its combination with 5-Methylcytidine, in terms of translation capacity. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Svitkin YV, Cheng YM, Chakraborty T, et al. N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Research, 2017, 45(10): 6023-6036. 2. Andries O, Mc Cafferty S, De Smedt SC, et al N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. Journal of Controlled Release, 2015, 217: 337-344. |
|
Quality Control & MSDS
- View current batch:
Chemical structure