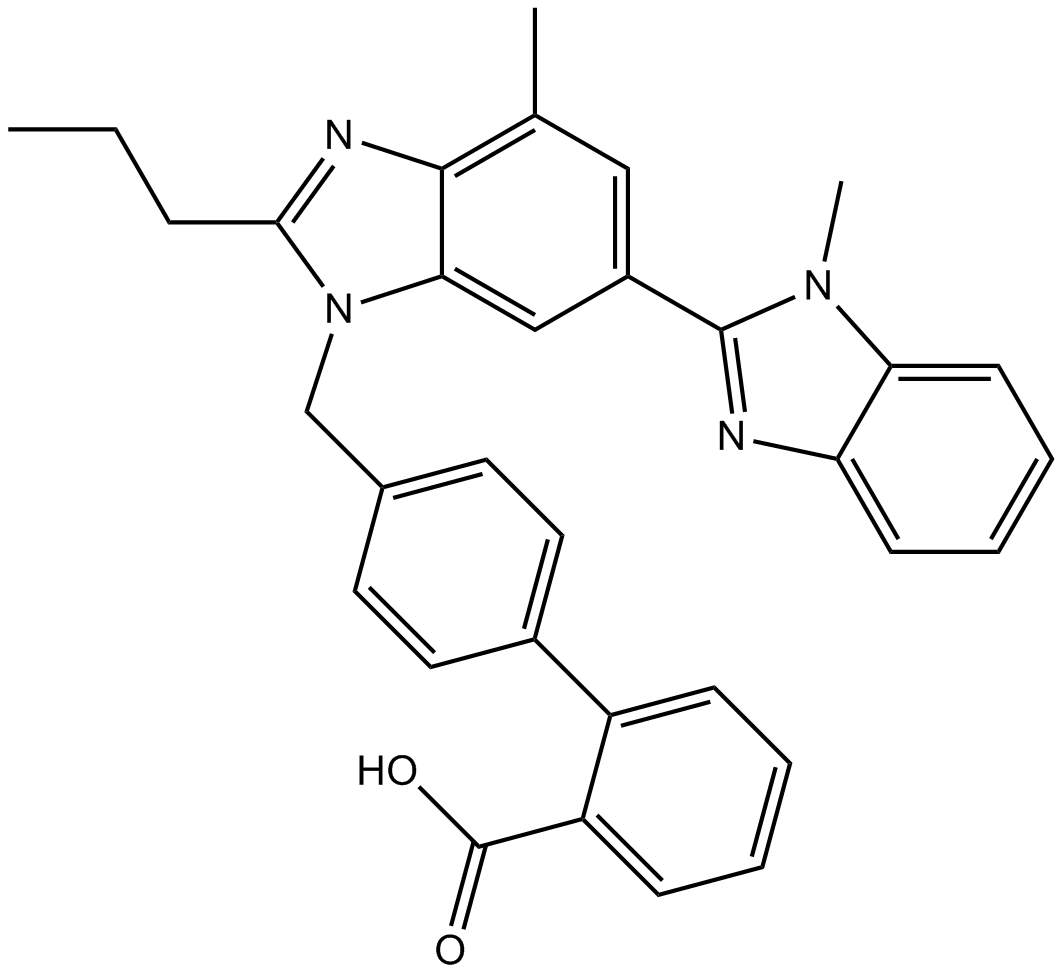
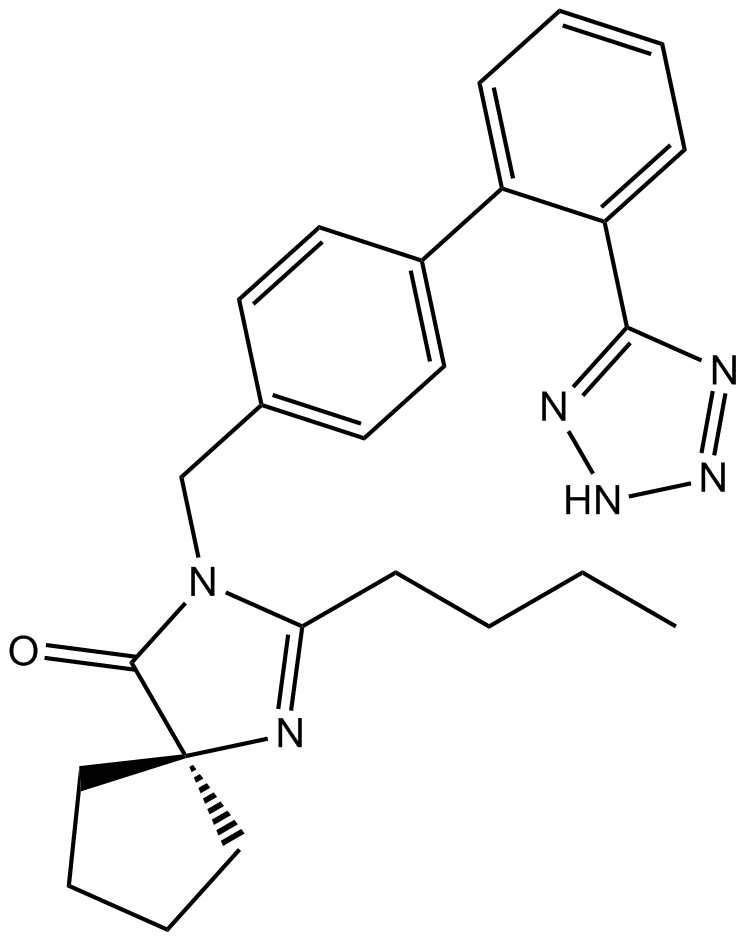
PD123319
PD123319 is a non-peptide inhibitor of angiotensin II receptor with IC50 value of 34nM [1].
Angiotensin II play roles in a variety of physiological functions. Among these, the most prominent is vascular contraction. Unlike previous drugs act as inhibitors of the formation of Ang II or ACE, PD123319 is an antagonist of angiotensin II receptor. PD123319 shows inhibition potency in both rat adrenal and brain binding assay with IC50 values of 34nM and 210nM, respectively. It is found to prevent Ang II from binding the bovine zona glomerulosa microsomal preparation with IC50 value of 6.9nM in the binding assay using microsome. In addition, it is reported that administration of PD123319 can suppress the generation of cyclic guanosine monophosphate and increase the production of prostaglandin E2. Besides that, administration of PD-123319 does not infiuence the effect of Ang II on protein tyrosine phosphorylation or thymidine incorporation [1, 2 and 3].
Reference:
[1] Blankley C J, Hodges J C, Klutchko S R, et al. Synthesis and structure-activity relationships of a novel series of non-peptide angiotensin II receptor binding inhibitors specific for the AT2 subtype. Journal of medicinal chemistry, 1991, 34(11): 3248-3260.
[2] Boulay G, Servant G, Luong T T, et al. Modulation of angiotensin II binding affinity by allosteric interaction of polyvinyl sulfate with an intracellular domain of the DuP-753-sensitive angiotensin II receptor of bovine adrenal glomerulosa. Molecular pharmacology, 1992, 41(4): 809-815.
[3] Siragy H. Angiotensin II receptor blockers: review of the binding characteristics. The American journal of cardiology, 1999, 84(10): 3-8.
| Physical Appearance | A colorless oil |
| Storage | Store at -20°C |
| M.Wt | 508.61 |
| Cas No. | 130663-39-7 |
| Formula | C31H32N4O3 |
| Solubility | ≥22.4 mg/mL in DMSO; ≥104.2 mg/mL in H2O; ≥140 mg/mL in EtOH |
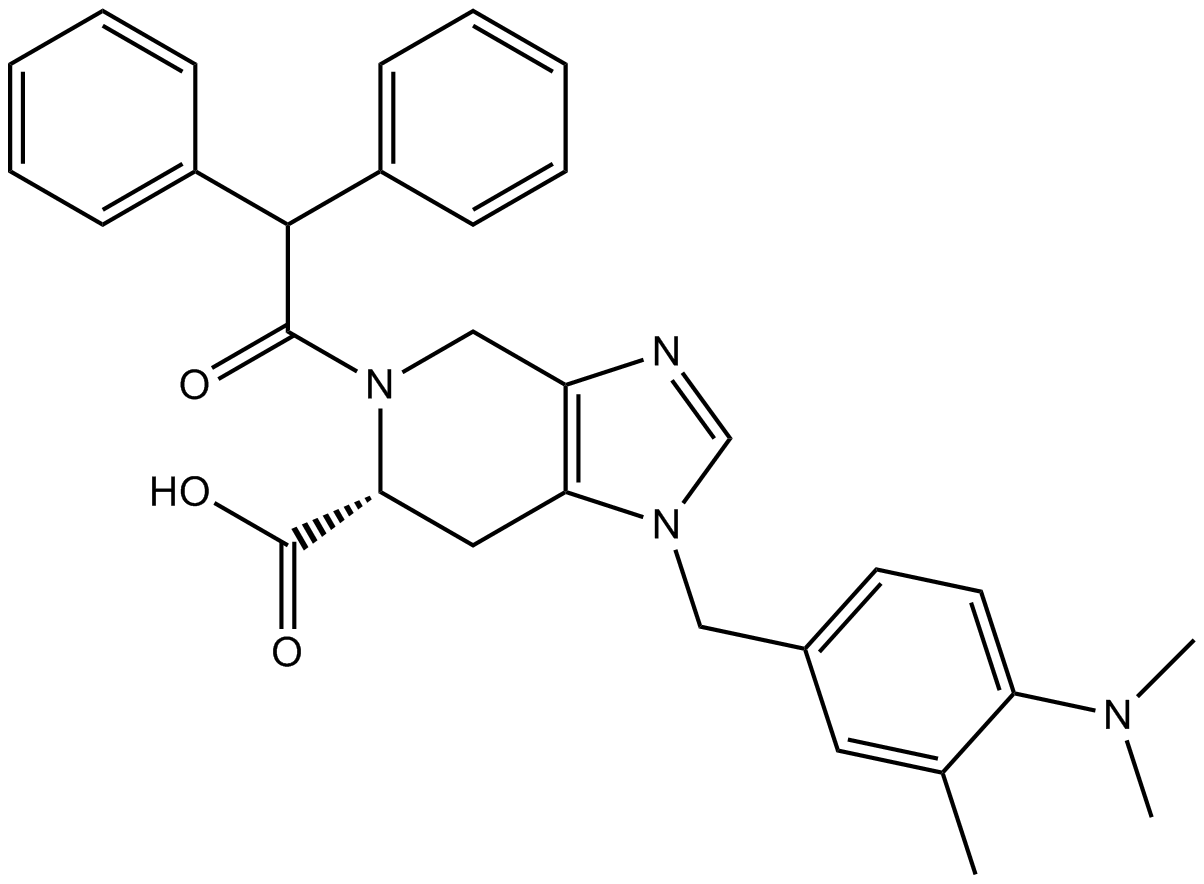
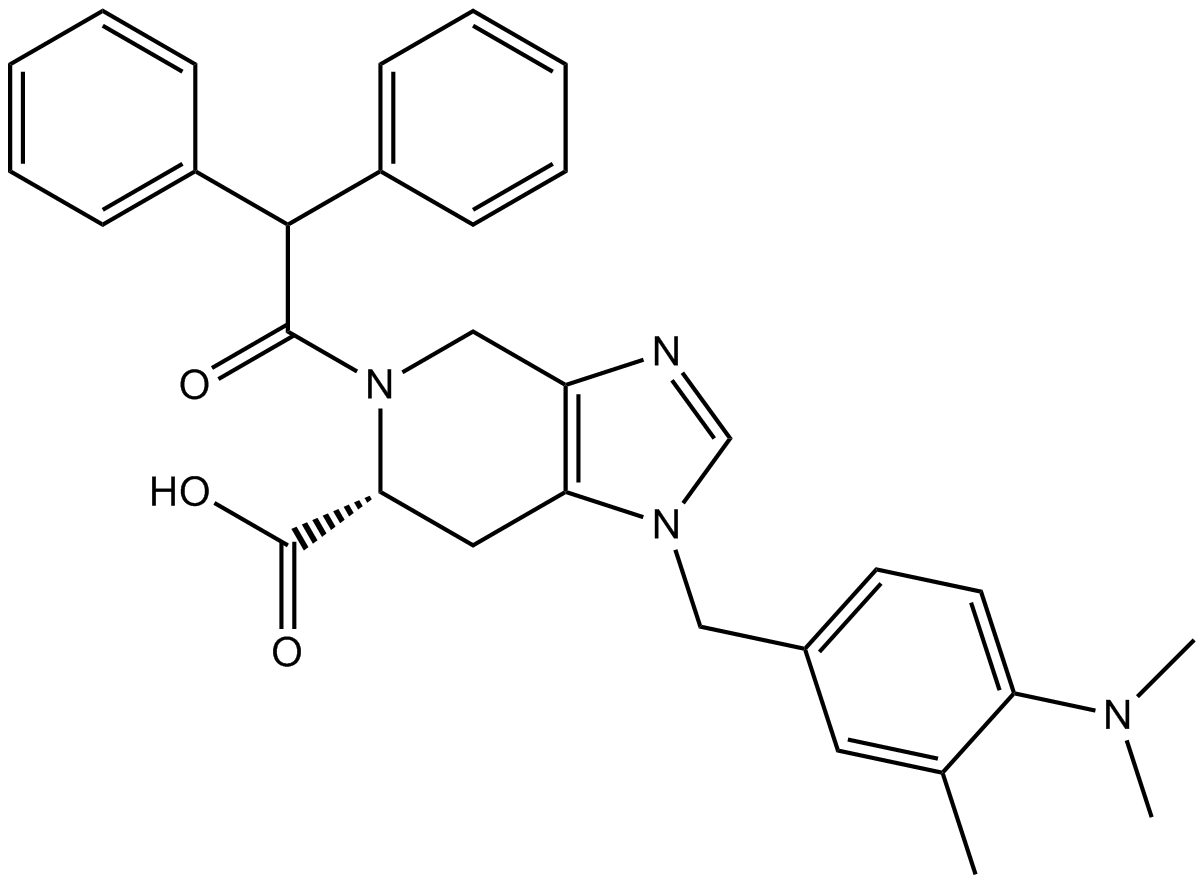
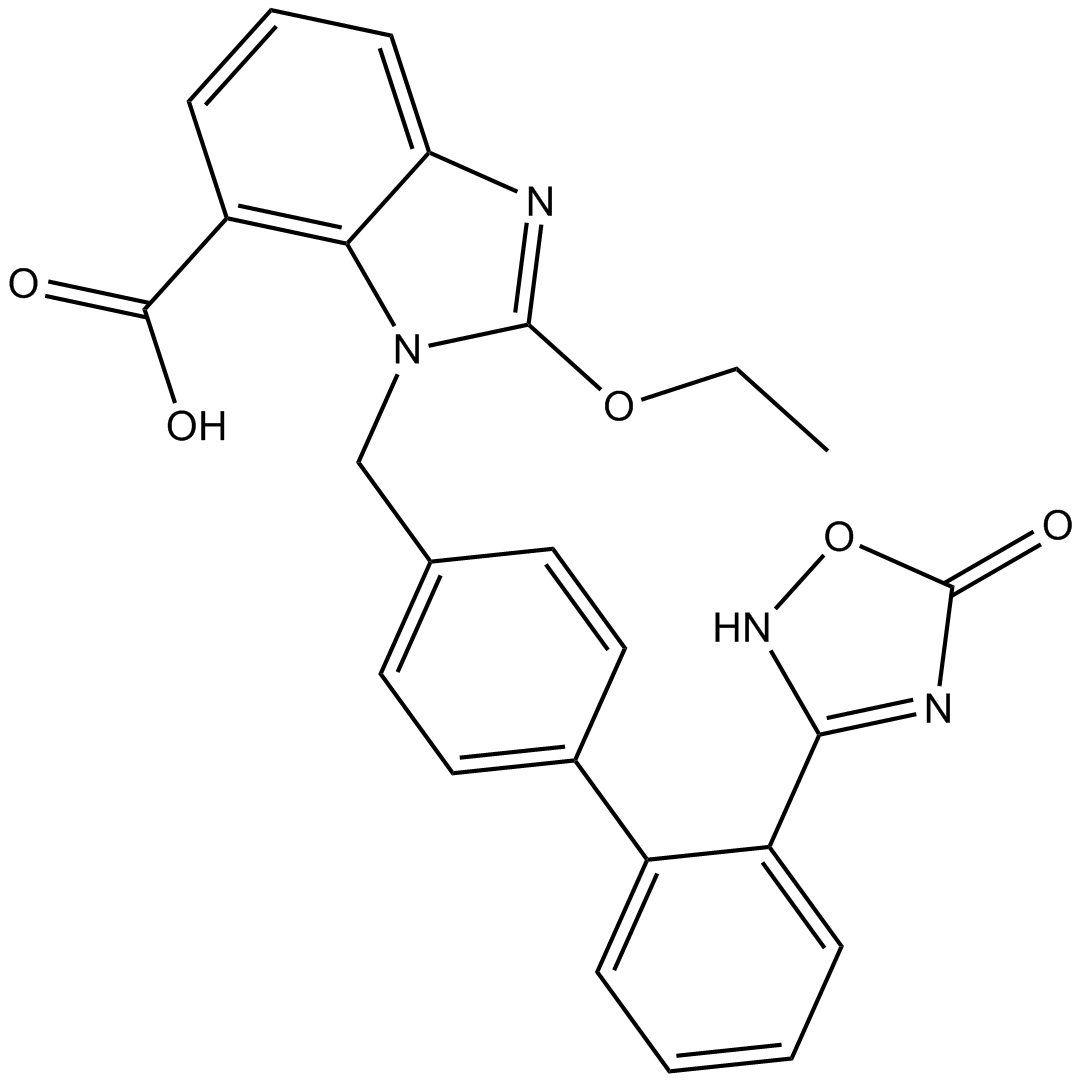
| Chemical Name | (6S)-1-[[4-(dimethylamino)-3-methylphenyl]methyl]-5-(2,2-diphenylacetyl)-6,7-dihydro-4H-imidazo[4,5-c]pyridine-6-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | CC1=C(C=CC(=C1)CN2C=NC3=C2CC(N(C3)C(=O)C(C4=CC=CC=C4)C5=CC=CC=C5)C(=O)O)N(C)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Human mesenchymal stem cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
10 μM for 15 days |
|
Applications |
PD123319 suppressed osteogenic differentiation of human mesenchymal stem cells through inhibition of extracellular signal-regulated kinase signaling. |
| Animal experiment [2]: | |
|
Animal models |
Rats model |
|
Dosage form |
0.5 or 2 mg/kg/day; subcutaneous injection for 6, 10 days |
|
Applications |
PD123319 attenuated hyperoxia-induced lung and heart injury at a low dose in newborn rats. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1Matsushita, K., Wu, Y., Pratt, R. E. and Dzau, V. J. (2015) Blockade of angiotensin II type 2 receptor by PD123319 inhibits osteogenic differentiation of human mesenchymal stem cells via inhibition of extracellular signal-regulated kinase signaling. J Am Soc Hypertens. 9, 517-525 2Wagenaar, G. T., Sengers, R. M., Laghmani el, H., Chen, X., Lindeboom, M. P., Roks, A. J., Folkerts, G. and Walther, F. J. (2014) Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats. Am J Physiol Lung Cell Mol Physiol. 307, L261-272 |
|
| Description | PD 123319 is a potent, selective antagonist of AT2 angiotensin II receptor with an IC50 value of 34 nM. | |||||
| Targets | AT2 receptor | |||||
| IC50 | 34 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure