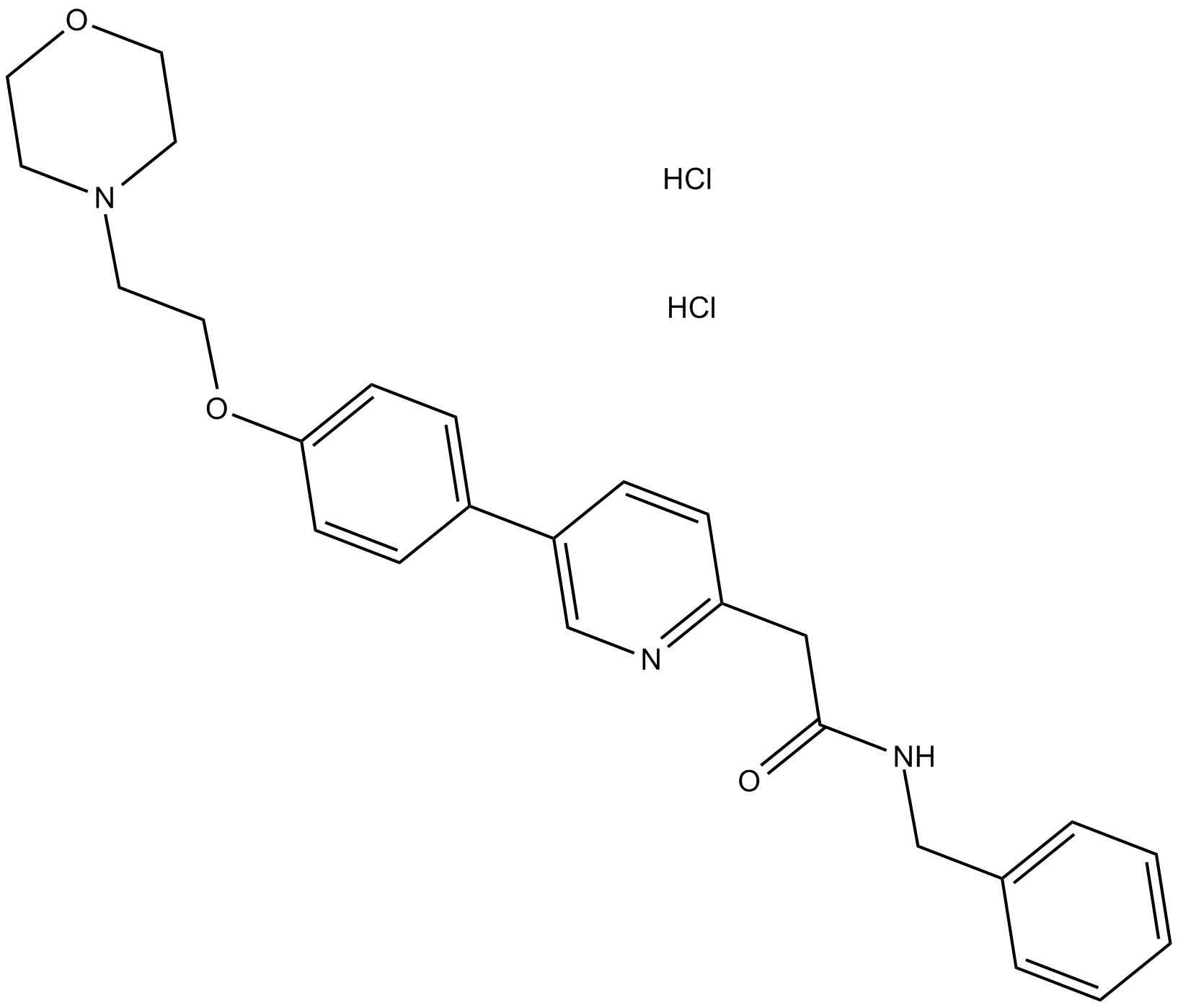
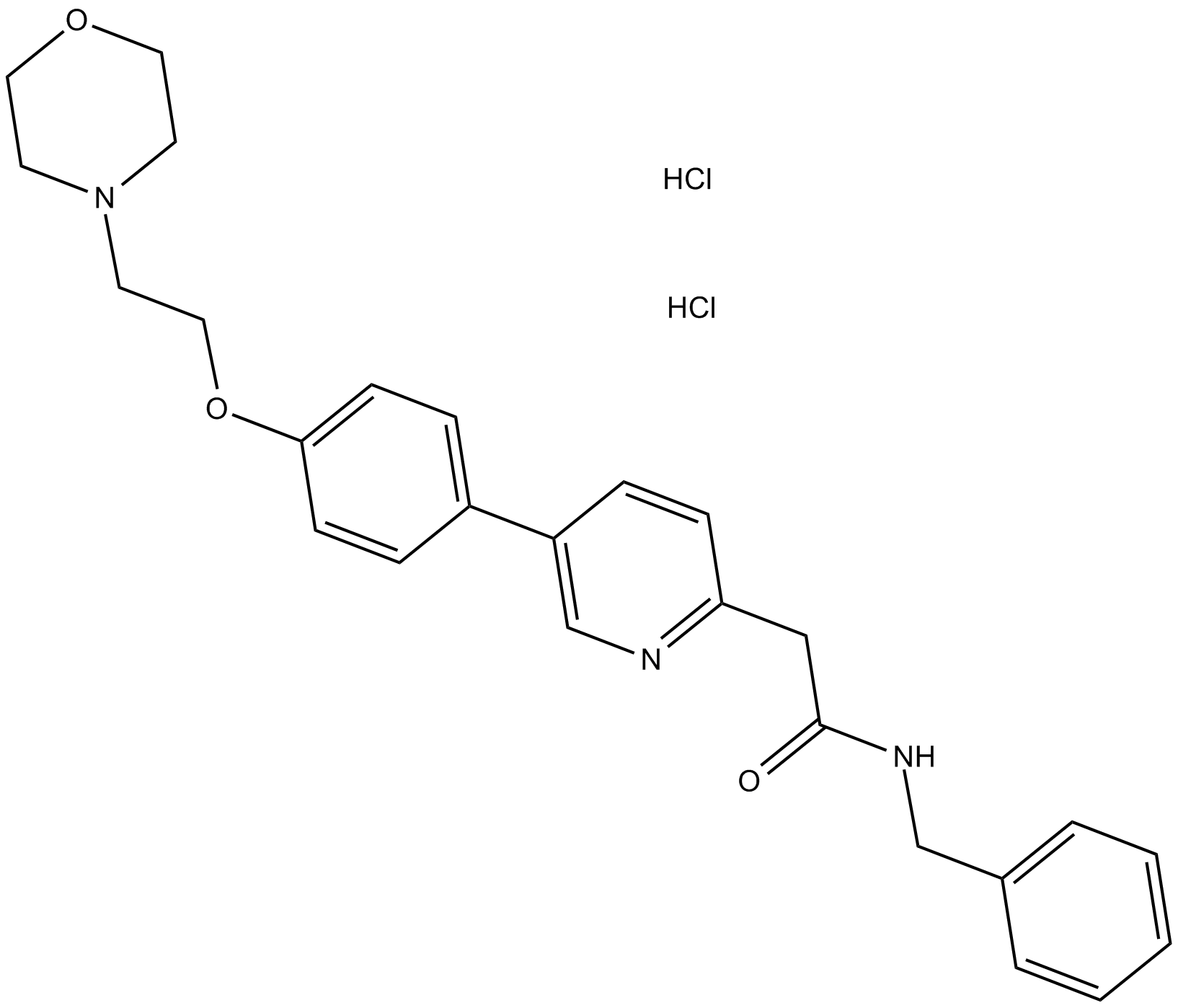
KX2-391 dihydrochloride
KX2-391 dihydrochloride (CAS No. 1038395-65-1) is a small-molecule inhibitor with a multi-target, dual mechanism of action. Its core bioactivities include inhibition of Src kinase, tubulin polymerization, hepatitis B virus (HBV) transcription, and botulinum neurotoxin A (BoNT/A) activity. Its targets are the Src kinase substrate-binding site, a novel binding site on the α-β tubulin heterodimer, the HBV precore promoter, and the BoNT/A light chain (LC). In terms of IC50/GI50, Src kinase inhibition is 23 nM in NIH3T3/c-Src527F cells and 39 nM in SYF/c-Src527F cells; inhibition of tubulin polymerization in cells requires ≥80 nM; anti-HBV EC50 values are 0.14 μM in PXB cells and 2.7 μM in HepG2-NTCP cells; anti-BoNT/A activity at 10-40 μM can inhibit SNAP-25 cleavage. Common application concentrations: in vitro cell experiments (anticancer / anti-HBV) 0.013-10 μM, anti-BoNT/A 10-40 μM; in animal experiments, mice are given 5-15 mg/kg orally (once or twice daily), chimpanzees for anti-HBV 1 mg/kg twice daily; in clinical use, 1% ointment (10 mg/g) for topical administration and 40-120 mg/day for oral tumor treatment. Effective therapeutic concentrations: for actinic keratosis, topical 1% ointment once daily for 5 consecutive days; for tumors, oral dosing maintains plasma peak concentrations of 61-218 ng/mL; for anti-HBV activity, plasma concentrations ≥560 nM (241.92 ng/mL) are required. The selectivity index for anti-HBV is 450 in PXB cells and > 37 in HepG2-NTCP cells. It shows good clinical tolerability with no obvious peripheral neuropathy.
References:
[1] Fallah-Tafti A, Foroumadi A, Tiwari R, Shirazi AN, Hangauer DG, Bu Y, Akbarzadeh T, Parang K, Shafiee A. Thiazolyl N-benzyl-substituted acetamide derivatives: synthesis, Src kinase inhibitory and anticancer activities. Eur J Med Chem. 2011 Oct;46(10):4853-8. doi: 10.1016/j.ejmech.2011.07.050. Epub 2011 Aug 4. PMID: 21852023.
[2] Harada K, Nishitsuji H, Ujino S, Shimotohno K. Identification of KX2-391 as an inhibitor of HBV transcription by a recombinant HBV-based screening assay. Antiviral Res. 2017 Aug;144:138-146. doi: 10.1016/j.antiviral.2017.06.005. Epub 2017 Jun 15. PMID: 28624460.
[3] Smolinski MP, Bu Y, Clements J, Gelman IH, Hegab T, Cutler DL, Fang JWS, Fetterly G, Kwan R, Barnett A, Lau JYN, Hangauer DG. Discovery of Novel Dual Mechanism of Action Src Signaling and Tubulin Polymerization Inhibitors (KX2-391 and KX2-361). J Med Chem. 2018 Jun 14;61(11):4704-4719. doi: 10.1021/acs.jmedchem.8b00164. Epub 2018 Apr 17. PMID: 29617135.
[4] Nardou K, Nicolas M, Kuttler F, Cisarova K, Celik E, Quinodoz M, Riggi N, Michielin O, Rivolta C, Turcatti G, Moulin AP. Identification of New Vulnerabilities in Conjunctival Melanoma Using Image-Based High Content Drug Screening. Cancers (Basel). 2022 Mar 19;14(6):1575. doi: 10.3390/cancers14061575. PMID: 35326726; PMCID: PMC8946509.
[5] Omar AM, Khayat MT, Ahmed F, Muhammad YA, Malebari AM, Ibrahim SM, Khan MI, Shah DK, Childers WE, El-Araby ME. SAR Probing of KX2-391 Provided Analogues With Juxtaposed Activity Profile Against Major Oncogenic Kinases. Front Oncol. 2022 May 20;12:879457. doi: 10.3389/fonc.2022.879457. PMID: 35669422; PMCID: PMC9166630.
[6] Chen X, Chen J, Feng W, Huang W, Wang G, Sun M, Luo X, Wang Y, Nie Y, Fan D, Wu K, Xia L. FGF19-mediated ELF4 overexpression promotes colorectal cancer metastasis through transactivating FGFR4 and SRC. Theranostics. 2023 Feb 22;13(4):1401-1418. doi: 10.7150/thno.82269. PMID: 36923538; PMCID: PMC10008733.
[7] Moore S, Kulkarni V, Moore A, Landes JR, Simonette R, He Q, Rady PL, Tyring SK. Tirbanibulin decreases cell proliferation and downregulates protein expression of oncogenic pathways in human papillomavirus containing HeLa cells. Arch Dermatol Res. 2024 Jul 5;316(7):455. doi: 10.1007/s00403-024-03205-8. PMID: 38967656.
[8] Koc D, Ibis K, Besarat P, Banoglu E, Kiris E. Tirbanibulin (KX2-391) analog KX2-361 inhibits botulinum neurotoxin serotype A mediated SNAP-25 cleavage in pre- and post-intoxication models in cells. Drug Dev Res. 2024 Sep;85(6):e22248. doi: 10.1002/ddr.22248. PMID: 39166850.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 504.45 |
| Cas No. | 1038395-65-1 |
| Formula | C26H31Cl2N3O3 |
| Synonyms | Tirbanibulin dihydrochloride; KX-01 dihydrochloride |
| Solubility | ≥25.2 mg/mL in DMSO; insoluble in H2O; ≥48.8 mg/mL in EtOH with gentle warming |
| Chemical Name | N-benzyl-2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]acetamide;dihydrochloride |
| SDF | Download SDF |
| Canonical SMILES | C1COCCN1CCOC2=CC=C(C=C2)C3=CN=C(C=C3)CC(=O)NCC4=CC=CC=C4.Cl.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure