Forskolin
Forskolin (CAS 66575-29-9) is a diterpenoid isolated from the plant Coleus forskohlii, known to directly activate adenylate cyclase type I, thereby elevating cellular cyclic AMP (cAMP) concentrations. It exhibits an IC50 of approximately 41 nM against adenylate cyclase. By increasing intracellular cAMP, forskolin modulates signaling pathways involved in inflammation and oxidative stress, reducing macrophage activation and subsequent production of thromboxane B2 and superoxide. Forskolin has been explored in various experimental models and clinical contexts, including cardiovascular disorders, diabetes mellitus, and asthma research.
References:
[1]. Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A, 1981, 78(6): 3363-3367.
[2]. Awad JA, Johnson RA, Jakobs KH, et al. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J Biol Chem, 1983, 258(5): 2960-2965.
[3]. Takeda J, Adachi K, Halprin KM, et al. Forskolin activates adenylate cyclase activity and inhibits mitosis in in vitro in pig epidermis. J Invest Dermatol, 1983, 81(3): 236-240.
- 1. Hyung Suk Oh, Shu-Fan Chou, et al. "Validation of human sensory neurons derived from inducible pluripotent stem cells as a model for latent infection and reactivation by herpes simplex virus 1." mBio. 2025 Aug 18:e0187125. PMID: 40823836
- 2. Ruize Shi, Mengyi Jin, et al. "Retinoic acid promotes conjunctival epithelium differentiation and goblet cell regeneration: evidence from novel 3D conjunctival organoids and whole-mount PAS staining." Ocul Surf.2025 Jul:37:301-313.
- 3. Hüseyin Gül, Jamie A Davies, et al. "Targeting TRPM3 as a potential therapeutic approach for autosomal dominant polycystic kidney disease." Sci Rep. 2025 Feb 8;15(1):4714. PMID: 39922884
- 4. Chan Wang, Yingyi Quan, al. "Protein Coronation-Induced Cancer Staging-Dependent Multilevel Cytotoxicity: An All-Humanized Study in Blood Vessel Organoids." ACS Nano. 2025 Jan14;19(1):345-368. PMID: 39743836
- 5. Sneha Kesaraju, Yueying Li, et al. "Claudin-2 limits pancreatitis development through regulating tight junction-controlled pancreatic ductal transport." bioRxiv. September 11, 2023
- 6. Lina Xu, Guoliang Wang, et al. "A cocktail of small molecules maintains the stemness and differentiation potential of conjunctival epithelial cells." Ocul Surf. 2023 Aug 25;S1542-0124(23)00109-X. PMID: 37634570
- 7. Blackford SJI, Yu TTL, et al. "RGD density along with substrate stiffness regulate hPSC hepatocyte functionality through YAP signalling." Biomaterials 2023 Feb;293 PMID: 36640555
- 8. Lu Gan, Qiyong Li, et al. "Glucocorticoids rapidly promote YAP phosphorylation via the cAMP-PKA pathway to repress mouse cardiomyocyte proliferative potential." Mol Cell Endocrinol. 2022 Mar 10;548:111615. PMID: 35278645
- 9. Xiaoya An, Guoliang Wang, et al. "Novel cell culture paradigm prolongs mouse corneal epithelial cell proliferative activity in vitro and in vivo." Front Cell Dev Biol. 2021 Jun 30;9:675998. PMID: 34277619
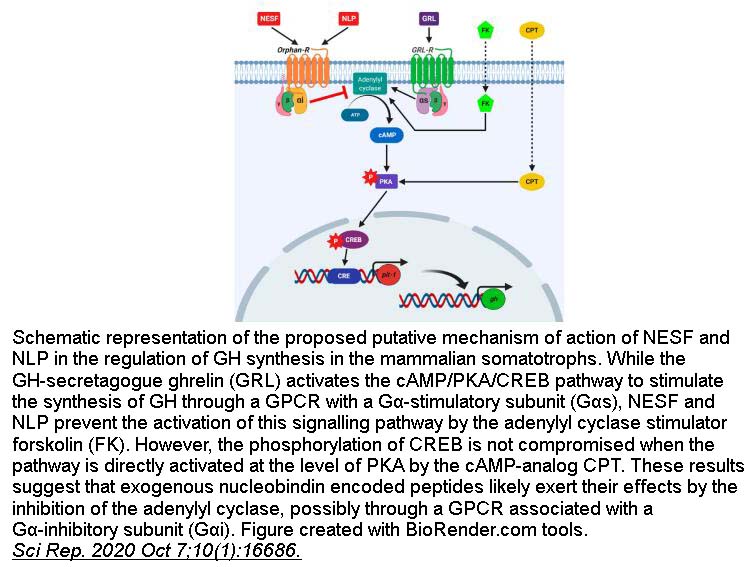
- 10. Emilio J Vélez, Suraj Unniappan. "Nesfatin-1 and nesfatin-1-like peptide suppress growth hormone synthesis via the AC/PKA/CREB pathway in mammalian somatotrophs." Sci Rep. 2020 Oct 7;10(1):16686. PMID: 33028951
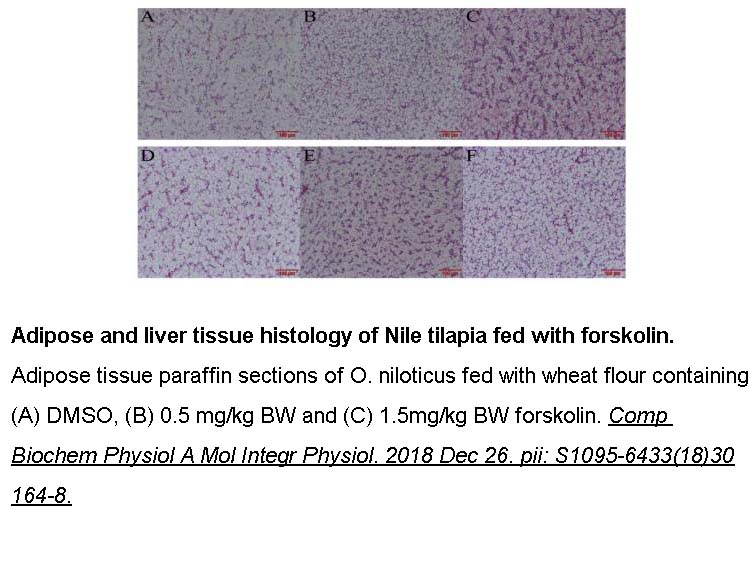
- 11. Liu DT, Hong WS, et al. "Upregulation of adamts9 by gonadotropin in preovulatory follicles of zebrafish." Mol Cell Endocrinol. 2019 Oct 2;499:110608. PMID: 31586455
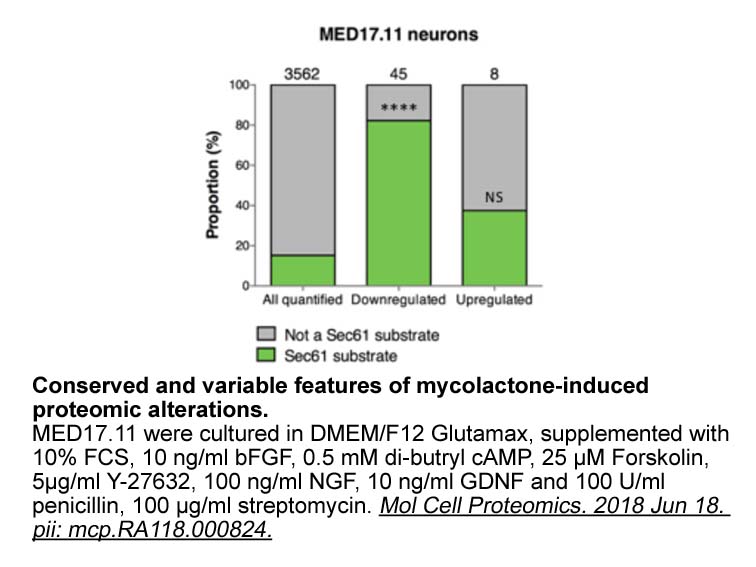
- 12. Zhang H, Wen JJ, et al. "Forskolin reduces fat accumulation in Nile tilapia (Oreochromis niloticus) through stimulating lipolysis and beta-oxidation." Comp Biochem Physiol A Mol Integr Physiol. 2018 Dec 26;230:7-15. PMID: 30593869
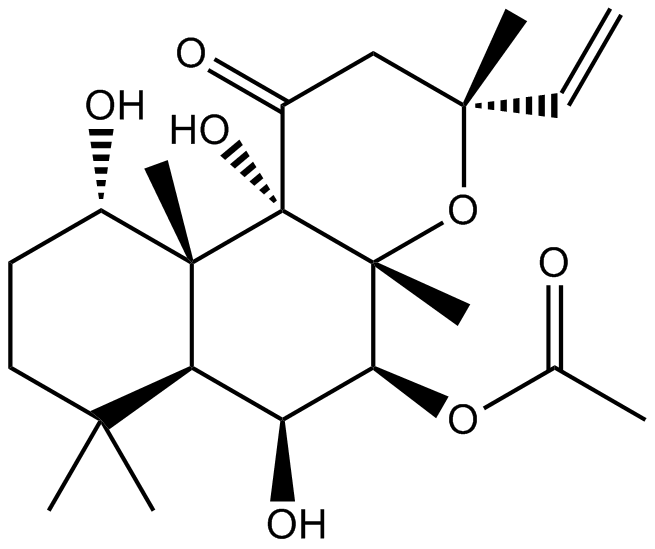
- 13. Morel JD, Paatero AO, et al. "Proteomics reveals scope of mycolactone-mediated Sec61 blockade and distinctive stress signature." Mol Cell Proteomics. 2018 Jun 18. pii: mcp.RA118.000824. PMID: 29915147
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 410.5 |
| Cas No. | 66575-29-9 |
| Formula | C22H34O7 |
| Solubility | insoluble in H2O; ≥13.43 mg/mL in EtOH; ≥20.53 mg/mL in DMSO |
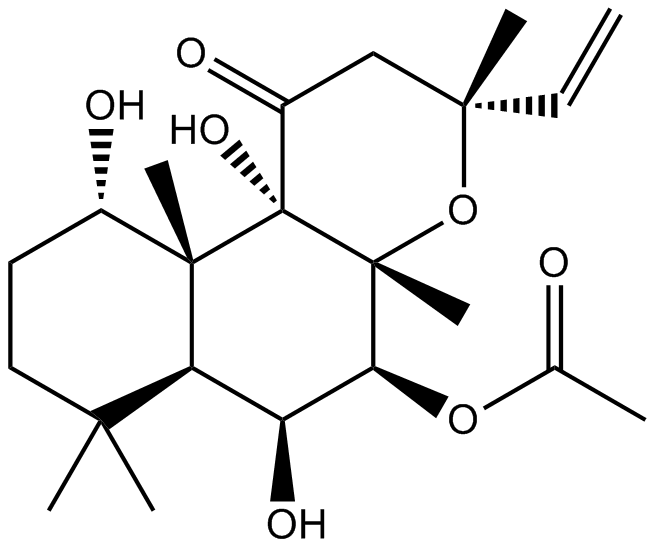
| Chemical Name | [(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-5-yl] acetate |
| SDF | Download SDF |
| Canonical SMILES | CC(C)(CC[C@@H]1O)[C@H]([C@@H]([C@@H]([C@@]2(C)O[C@](C)(CC3=O)C=C)OC(C)=O)O)[C@@]1(C)[C@]23O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1, 2]: | |
|
Cell lines |
Human mesenchymal stem cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0.075-0.2 mM for 4 days or 7 days; or 10 μM |
|
Applications |
Forskolin concentration-dependently decreased human mesenchymal stem cells proliferation after 4 days. Moreover, Forskolin increased alkaline phosphatase (ALP) expression of human mesenchymal stem cells in a dose-dependent manner. Additionally, Forskolin (10 μM) significantly stimulated both vasopressin and oxytocin release from the rat hypothalamo-neurohypophysial (H-NH) system. |
| Animal experiment [1]: | |
|
Animal models |
Particles were implanted in subcutaneous pockets of nude male mice model |
|
Dosage form |
0.10 or 0.15mM Forskolin |
|
Applications |
Treatment with 0.10 mM Forskolin enhanced bone formation by human mesenchymal stromal cells in vivo. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1. Doorn, J., Siddappa, R., van Blitterswijk, C. A. and de Boer, J. (2012) Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng Part A. 18, 558-567 2. Roszczyk, M. and Juszczak, M. (2014) Forskolin-stimulated vasopressin and oxytocin release from the rat hypothalamo-neurohypophysial system in vitro is inhibited by melatonin. Endokrynol Pol. 65, 125-131 |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data