Doxycycline hyclate
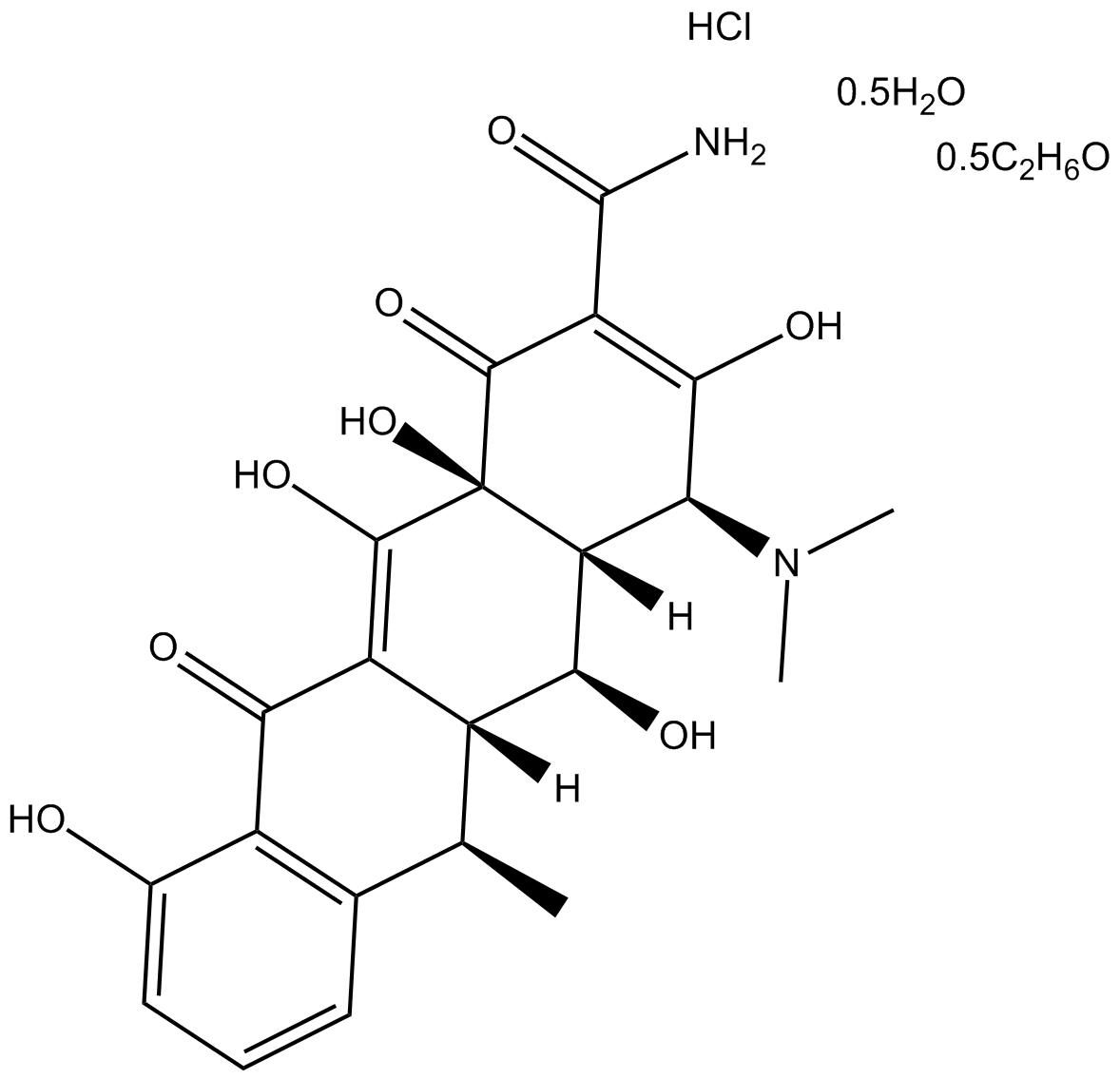
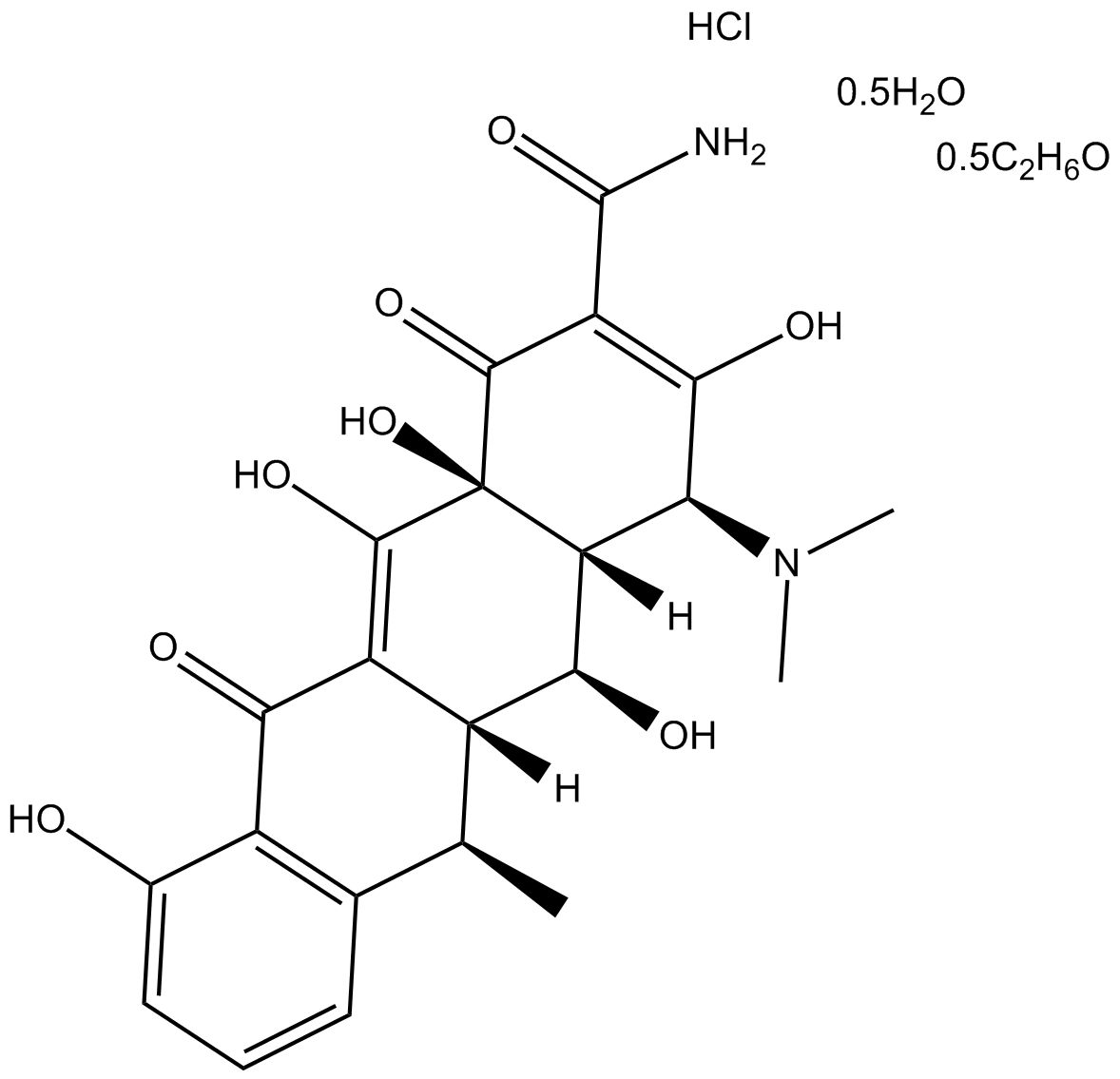
Doxycycline hyclate (CAS 24390-14-5)is a semisynthetic antibiotic derivative of tetracycline, exhibiting antibacterial, antiviral and anti-inflammatory activities. It functions primarily as an inhibitor of matrix metalloproteinases (MMPs), notably reducing expression and activity of MMP-2, MMP-8, and MMP-9, thus applicable in research involving inflammatory conditions and vascular pathologies, such as intracranial aneurysms. Its antiviral properties include inhibition of dengue virus replication by targeting viral NS2B-NS3 serine protease; experimental conditions at 37°C and 40°C yielded IC50 values of 52.3 μM and 26.7 μM respectively. Additionally, doxycycline hyclate demonstrates antimalarial activity, with in vivo experiments showing an IC50 of 320 nM at 96 hours, facilitating potential use in parasitology research models.
References:
[1] Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol. 2014 Apr;159(4):711-8.
[2] Maradni A, Khoshnevisan A, Mousavi SH, Emamirazavi SH, Noruzijavidan A. Role of matrix metalloproteinases (MMPs) and MMP inhibitors on intracranial aneurysms: a review article. Med J Islam Repub Iran. 2013 Nov;27(4):249-254.
[3] Draper MP, Bhatia B, Assefa H, Honeyman L, Garrity-Ryan LK, Verma AK, Gut J, Larson K, Donatelli J, Macone A, Klausner K, Leahy RG, Odinecs A, Ohemeng K, Rosenthal PJ, Nelson ML. In vitro and in vivo antimalarial efficacies of optimized tetracyclines. Antimicrob Agents Chemother. 2013 Jul;57(7):3131-6.
- 1. Kannan V. Manian, Connor H. Ludwig, et al. "A comprehensive map of missense trafficking variants in rhodopsin and their response to pharmacologic correction." bioRxiv. 2025 Mar 4:2025.02.27.640335. PMID: 40093169
- 2. Yuting Li, Ziqiong Yang, et al. "Prdm14 promotes mouse ESC self-renewal and PGCLC specification through enhancement of Stat3 activity." iScience. 2022 Oct 6;25(11):105293. PMID: 36300005
| Storage | Store at 4°C |
| M.Wt | 512.94 |
| Cas No. | 24390-14-5 |
| Formula | C23H29ClN2O9 |
| Solubility | ≥22.15 mg/mL in DMSO; insoluble in EtOH; ≥49.2 mg/mL in H2O with ultrasonic |
| SDF | Download SDF |
| Canonical SMILES | O=C(C(C1=O)=C(O)[C@@H](N(C)C)[C@]2([H])[C@@H](O)[C@]3([H])[C@@H](C)C4=C(C(C3=C(O)[C@@]21O)=O)C(O)=CC=C4)N.Cl.[0.5H2O].[0.5C2H6O] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
cultured P. falciparum parasites |
|
Preparation method |
The solubility of this compound in DMSO is >22.2mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
96-h |
|
Applications |
In cultured P. falciparum parasites, Doxycycline demonstrated nanomolar antimalarial activity with IC50 value of 330 nM. |
| Animal experiment [1]: | |
|
Animal models |
mouse model of P. berghei malaria |
|
Dosage form |
10 mg/kg and 50 mg/kg once daily; dissolved in PBS in a total volume of 100 μl; intraperitoneally |
|
Application |
In mouse model of P. berghei malaria, Doxycycline exhibited potent antimalarial activity at 50 mg/kg and activity was suboptimal at 10 mg/kg. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Draper MP, Bhatia B, Assefa H, Honeyman L, Garrity-Ryan LK, Verma AK, Gut J, Larson K, Donatelli J, Macone A, Klausner K, Leahy RG, Odinecs A, Ohemeng K, Rosenthal PJ, Nelson ML. In vitro and in vivo antimalarial efficacies of optimized tetracyclines. Antimicrob Agents Chemother. 2013 Jul;57(7):3131-6. |
|
Quality Control & MSDS
- View current batch:
Chemical structure