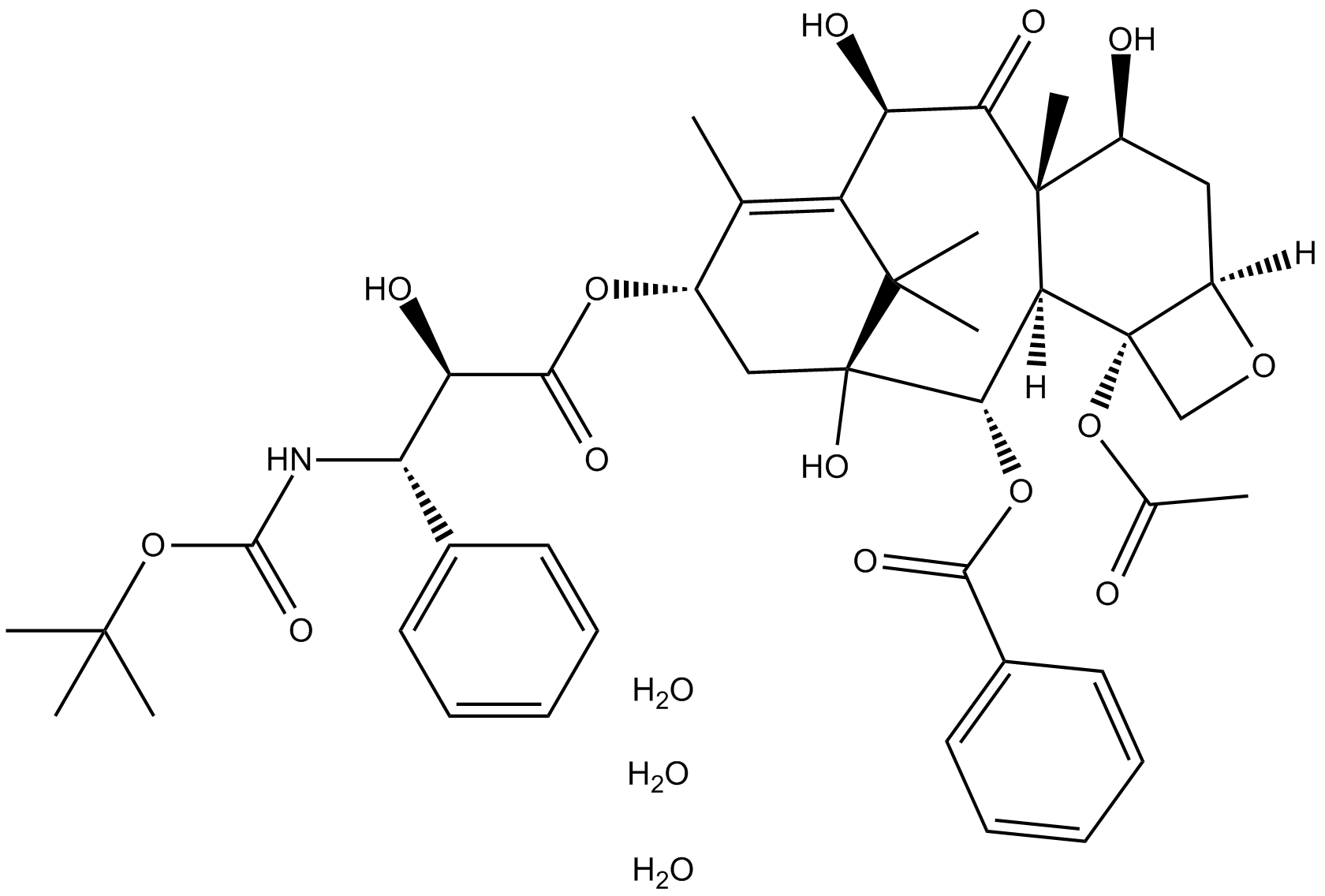
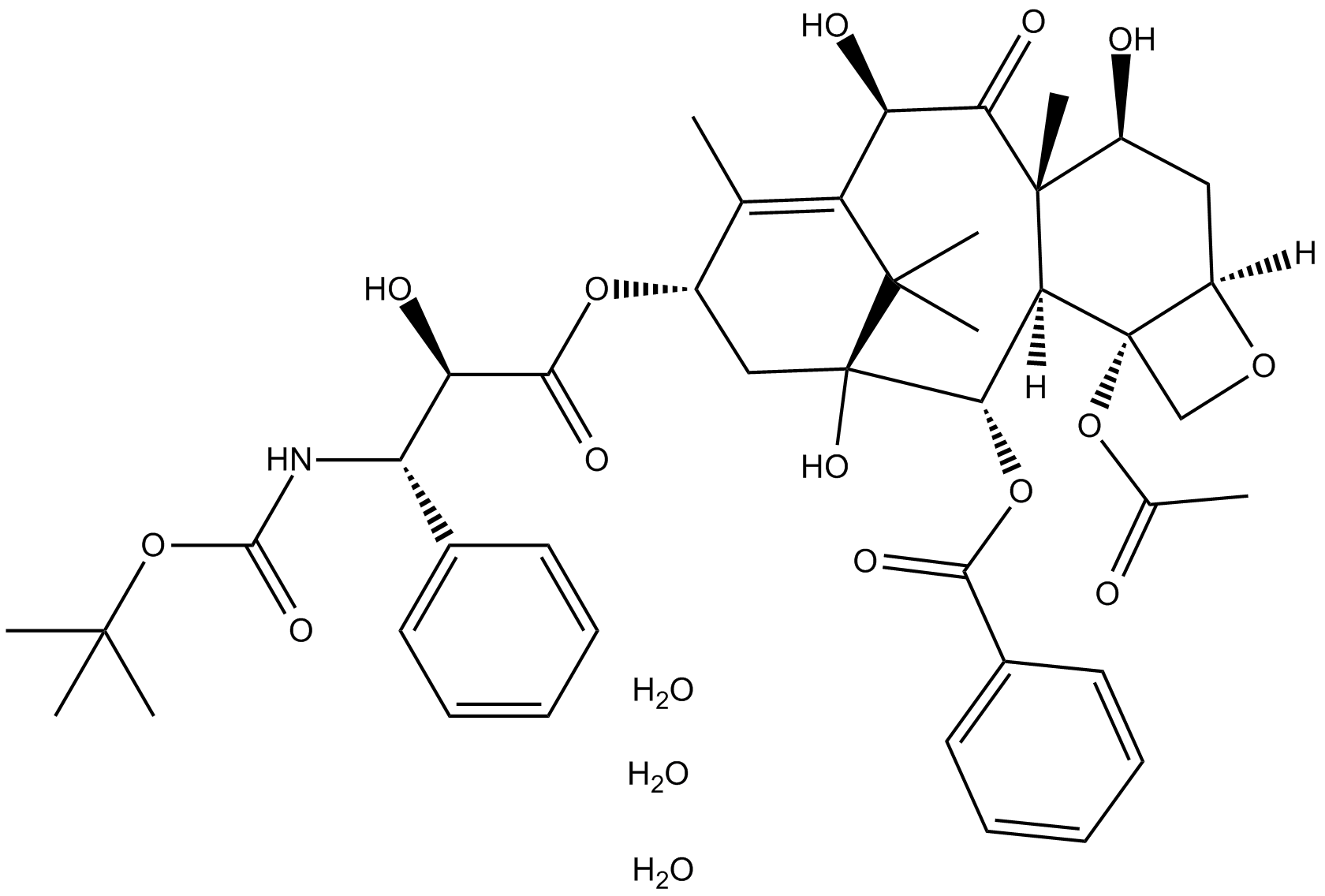
Docetaxel Trihydrate
Catalog No.
A3370
Depolymerisation of microtubules inhibitor
Featured Products
Description:
IC50 Value: N/A
Docetaxel, an analog of taxol, is an inhibitor of depolymerisation of microtubules through binding to stabilized microtubules. Docetaxel is a clinically well-established anti-mitotic chemotherapy medication. It is used mainly for the treatment of breast, ovarian, prostate, and non-small cell lung cancer.
in vitro: IC50 concentrations (reducing survival by 50%) ranged from 0.13-3.3 ng/ml, with three neuroblastoma lines proving most sensitive and three breast and two colon carcinoma lines showing least sensitivity [1]. Docetaxel was shown to promote the assembly of microtubule protein without GTP in vitro, but no inhibitory effect on DNA, RNA and protein synthesis [2]. Gene expression changes induced by paclitaxel treatment were mainly enriched in actin cytoskeleton (ACTC1, MYL2 and MYH2), tyrosine-protein kinases (ERRB4, KIT and TIE1) and focal adhesion pathway (MYL2, IGF1 and FLT1), while the expression alterations responding to docetaxel were highly co-related to cell surface receptor linked signal transduction (SHH, DRD5 and ADM2), cytokine-cytokine receptor interaction (IL1A and IL6) and cell cycleregulation (CCNB1, CCNE2 and PCNA) [4].
in vivo: The patients, between 15 and 80 years old with performance status (PS) of 0-2, received at least two cycles of docetaxel 60 mg m-2 intravenously at 3-4 week intervals [3]. Intestinal damage after repeated dosing of docetaxel (20 mg/kg) for 3 weeks was more severe at 14HALO than at 2HALO (hours after light on). The intestinal protein expressions of Wee1, phosphorylated CDK1, and cleaved Caspase-3 were higher in the 14HALO group than in the 2HALO group, while that of survivin was lower in the 14HALO group [5].
Toxicity: Twenty-five patients were enrolled. Overall, 13/25 (52 %, 95 % CI 34-70) completed 4 cycles, and 19/25 (76 %, 95 % CI 60-87) completed ≥3 cycles. Twenty of 25 patients (80 %) experienced a Grade 3 or 4 adverse event [6].
Clinical trial: N/A
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 861.93 |
| Cas No. | 148408-66-6 |
| Formula | C43H59NO17 |
| Synonyms | Taxotere Trihydrate |
| Solubility | ≥43.1 mg/mL in DMSO; insoluble in H2O; ≥87.6 mg/mL in EtOH with gentle warming |
| Chemical Name | (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2 |
| SDF | Download SDF |
| Canonical SMILES | O=C(OC(C)(C)C)N[C@H]([C@H](C(O[C@@H]1C(C)=C([C@@H](O)C([C@@]2(C)[C@]([C@@](CO3)(OC(C)=O)[C@@]3([H])C[C@@H]2O)([H])[C@@H]4OC(C5=CC=CC=C5)=O)=O)C(C)(C)[C@@]4(O)C1)=O)O)C6=CC=CC=C6.O.O.O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure