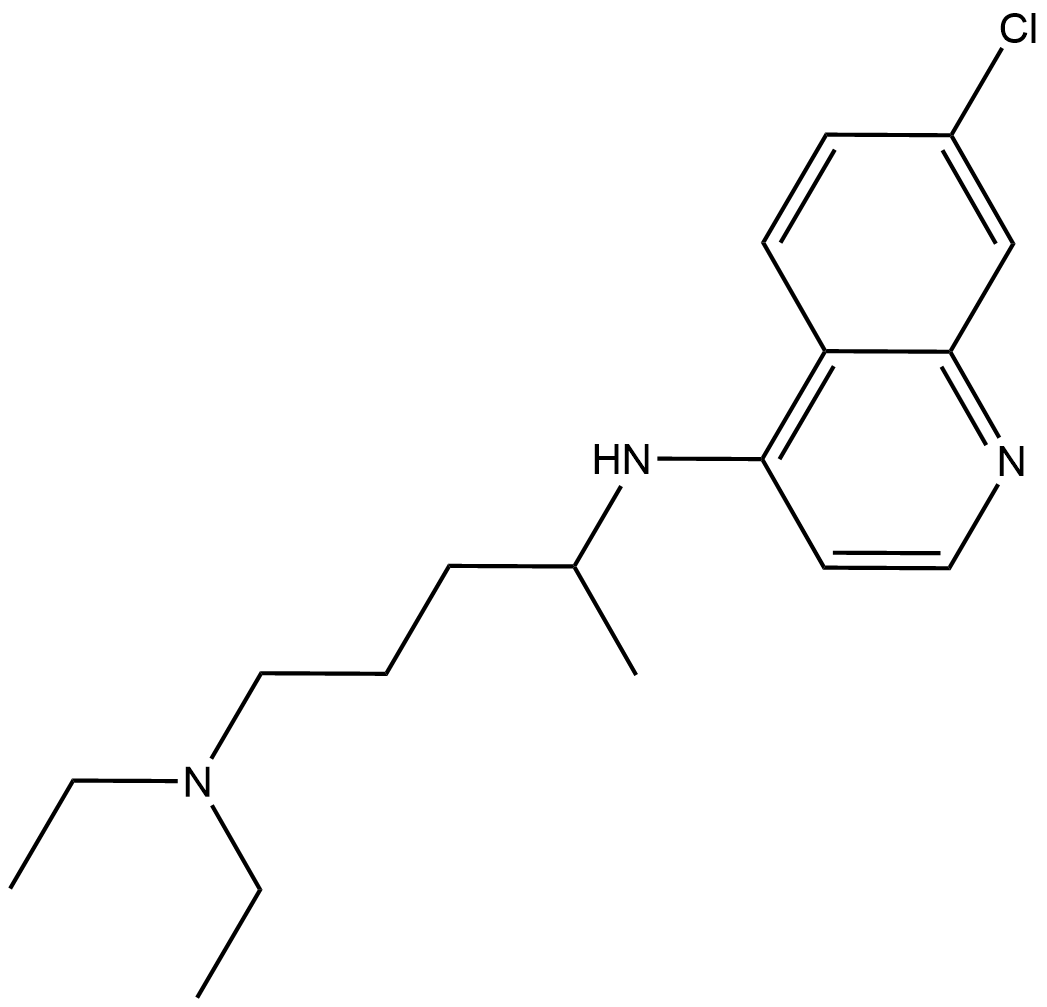
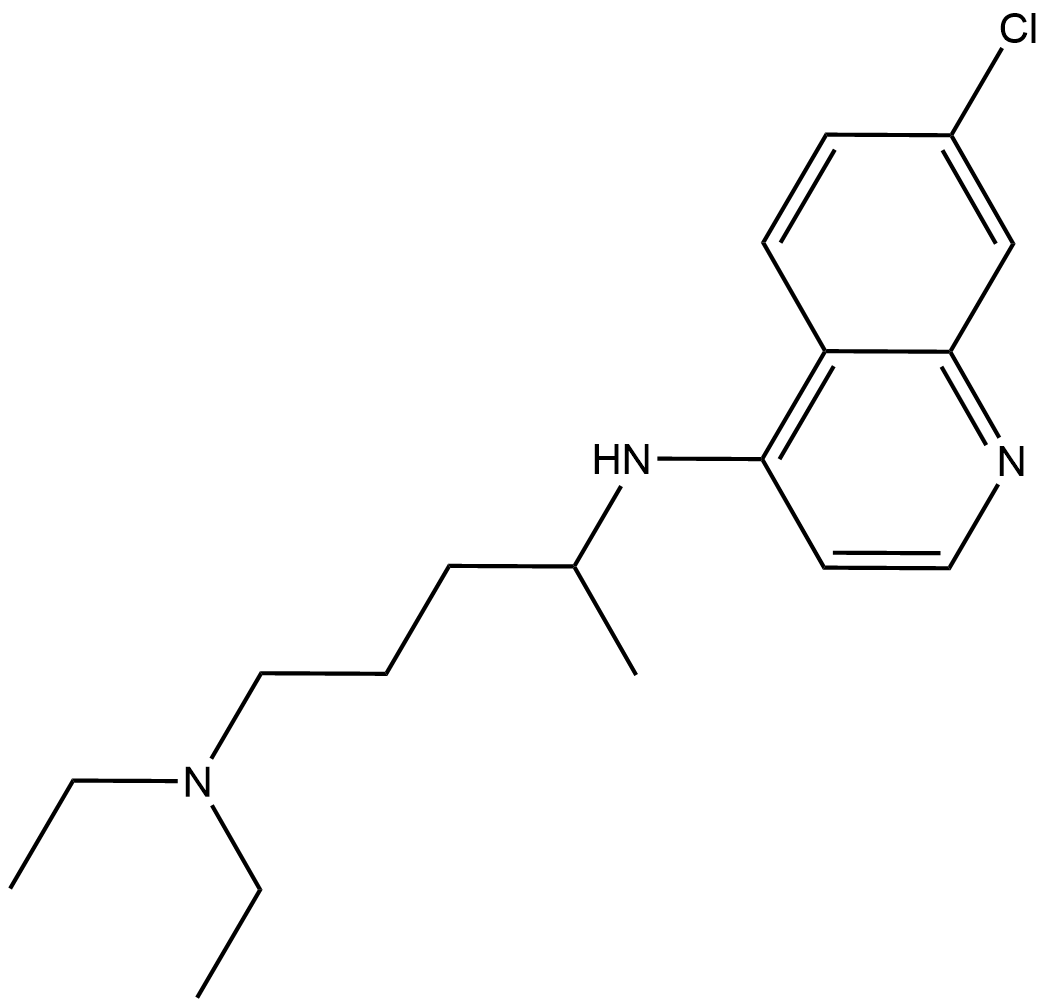
Chloroquine
Chloroquine (CAS No. 54-05-7) is a 4-aminoquinoline compound. Its core targets include lysosomes (elevating lysosomal pH to inhibit autophagy), p53 protein, the PI3K/AKT/mTOR signaling pathway, and toll-like receptors 3/7/9 (TLR3/7/9). Meanwhile, it can inhibit the glycosylation of viral receptors (e.g., ACE2) and plasmodial heme polymerase, and also affect drug efficacy by regulating metabolism mediated by CYP2C8/CYP3A4/CYP2D6.
Its activity concentration data are clearly defined. In the anticancer field, the 72-hour half-maximal inhibitory concentration (IC₅₀) values against ovarian cancer cell lines IGROV-1, OVCAR-8, SKOV-3, and A2780 are 29.05 μM, 28.25 μM, 22.28 μM, and 12.31 μM, respectively. When combined with lidamycin, the IC₅₀ against lung cancer A549 cells is 71.3±6.1 μM. The commonly used concentrations for colon cancer cell lines HT-29 and SW480 are 80 μM and 20 μM, respectively. In the antiviral field, it exhibits in vitro inhibitory activity against viruses such as SARS-CoV-2 and HIV-1. The commonly used concentration for in vitro cell experiments ranges from 5 to 80 μM, covering anticancer, anti-inflammatory, and antiviral research.
The clinically effective therapeutic concentration corresponds to multi-scenario dosages: for anticancer monotherapy, the oral dose is 150–250 mg/day; when combined with chemotherapy or radiotherapy, the dosage can be increased to an immediate loading dose of 500 mg plus a maintenance dose of 250 mg/day. Conventional oral doses are applied for antimalarial treatment and autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, SLE). In COVID-19 clinical trials, the oral dose is mostly 200–600 mg/day.
Its biological activities span multiple fields: it exerts broad-spectrum anticancer effects by inhibiting autophagy or inducing lysosomal/mitochondrial membrane permeability (LMP/MOMP); treats autoimmune diseases by downregulating the TLR signaling pathway; and blocks viral entry and replication to exert antiviral effects. Clinically, attention should be paid to adverse reactions such as renal impairment and cardiovascular toxicity. Nano-formulations can reduce toxicity and enhance targeting ability.
References:
[1] Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762. Epub 2020 Mar 5. PMID: 32147496; PMCID: PMC7132364.
[2] Biswas M, Sukasem C. Pharmacogenomics of chloroquine and hydroxychloroquine: current evidence and future implications. Pharmacogenomics. 2023 Oct;24(15):831-840. doi: 10.2217/pgs-2023-0124. Epub 2023 Oct 17. PMID: 37846548.
[3] Liu Y, Meng Y, Zhang J, Gu L, Shen S, Zhu Y, Wang J. Pharmacology Progresses and Applications of Chloroquine in Cancer Therapy. Int J Nanomedicine. 2024 Jul 5;19:6777-6809. doi: 10.2147/IJN.S458910. PMID: 38983131; PMCID: PMC11232884.
| Physical Appearance | A solid |
| Storage | 4°C, protect from light |
| M.Wt | 319.87 |
| Cas No. | 54-05-7 |
| Formula | C18H26ClN3 |
| Solubility | ≥20.8 mg/mL in DMSO; ≥32 mg/mL in EtOH; insoluble in H2O |
| Chemical Name | N4-(7-chloroquinolin-4-yl)-N1,N1-diethylpentane-1,4-diamine |
| SDF | Download SDF |
| Canonical SMILES | CC(CCCN(CC)CC)NC1=C2C(C=C(Cl)C=C2)=NC=C1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure