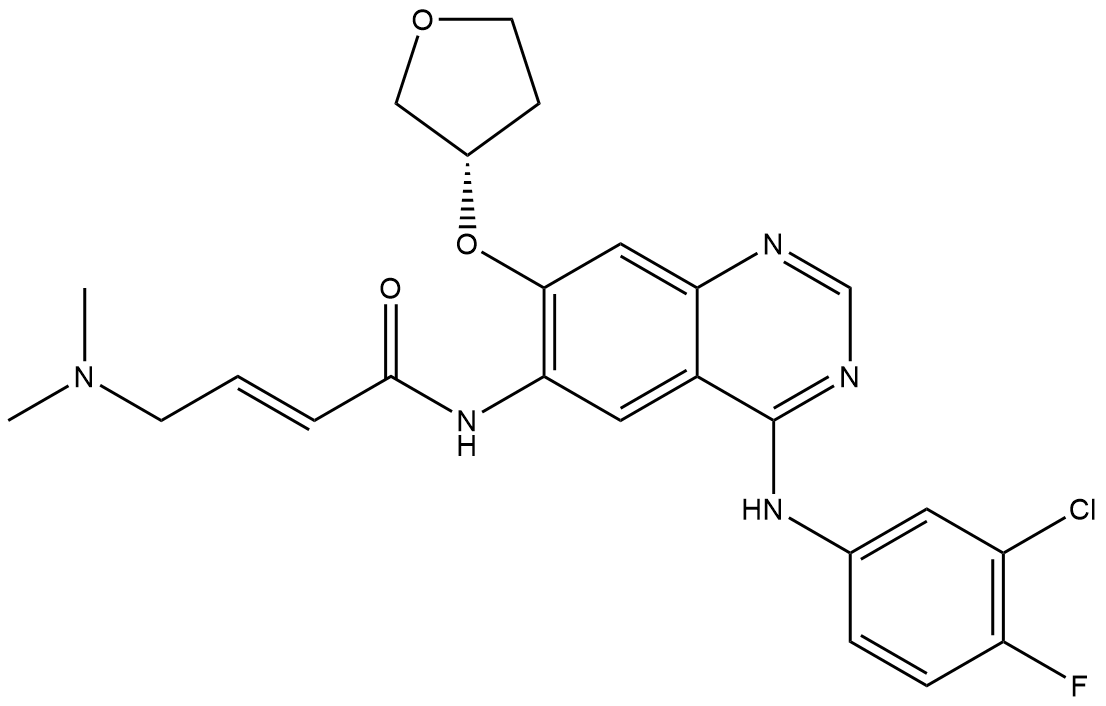
Afatinib
Catalog No.
A4746
A covalent inhibitor targeting members of the ErbB family
Featured Products
Afatinib is an irreversible, small-molecule tyrosine kinase inhibitor designed for research use. It serves as a critical pharmacological tool in oncology and signal transduction studies. It acts as a potent, covalent inhibitor targeting members of the ErbB family, primarily EGFR (ErbB1), HER2 (ErbB2), and HER4 (ErbB4). By forming a permanent covalent bond with the kinase domains, it irreversibly blocks their enzymatic activity and downstream pro-survival signaling pathways (e.g., MAPK, PI3K/Akt). This mechanism is effective even against certain resistant mutants, such as the T790M mutation in EGFR.
- 1. Irit Shapira-Netanelov, Olga Furman, et al. "Patient-Derived Gastric Cancer Assembloid Model Integrating Matched Tumor Organoids and Stromal Cell Subpopulations." Cancers (Basel). 2025 Jul 9;17(14):2287 PMID: 40723172
- 2. Yuhan Ding, Yao Yan, et al. "NRG1-ErbB4 signaling in the cerebrospinal fluid-contacting nucleus regulates thermal pain in mice." Neuroscience. 2025 Feb 6:566:132-141. PMID: 39733821
| Storage | Store at -20°C |
| M.Wt | 485.94 |
| Cas No. | 850140-72-6 |
| Formula | C24H25ClFN5O3 |
| Synonyms | BIBW 2992 |
| Solubility | ≥49.3 mg/mL in DMSO; ≥13.07 mg/mL in EtOH with ultrasonic; insoluble in H2O |
| Chemical Name | (S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-4-(dimethylamino)but-2-enamide |
| SDF | Download SDF |
| Canonical SMILES | CN(C)C/C=C/C(Nc(c(O[C@@H]1COCC1)c1)cc2c1ncnc2Nc(cc1)cc(Cl)c1F)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure