3-Deazaadenosine
3-Deazaadenosine (CAS 6736-58-9) is an inhibitor of S-adenosylhomocysteine (SAH) hydrolase, an enzyme responsible for the reversible hydrolysis of SAH into adenosine and homocysteine. Inhibition of SAH hydrolase by 3-Deazaadenosine (Ki = 3.9 μM) elevates intracellular SAH concentrations, altering the SAH-to-SAM (S-adenosylmethionine) ratio, and consequently suppressing SAM-dependent methyltransferase activities. In vitro studies demonstrate its antiviral activity against Ebola and Marburg viruses in various primate and mouse cell lines. Animal studies indicate protective efficacy against lethal Ebola infection in BALB/c mice. Currently, 3-Deazaadenosine remains in preclinical research.
- 1. Weiyun Wu, Xiaowen Li, et al. "METTL14 regulates inflammation in ulcerative colitis via the lncRNA DHRS4-AS1/miR-206/A3AR axis." Cell Biol Toxicol 40, 95 (2024).
- 2. Xinming Yin, Shijie Zhao, et al. "m6A-modified PADI2 facilitates proliferation and Cisplatin-resistance of epithelial ovarian cancer." Gynecol Oncol. 2024 Jan:180:99-110. PMID: 38086167
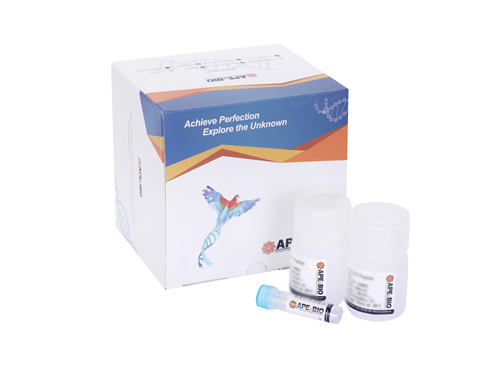
- 3. Li-Jie Chen, Hui-Ye Liu, et al. "IGF2BP3 promotes the progression of colorectal cancer and mediates cetuximab resistance by stabilizing EGFR mRNA in an m6A-dependent manner." Cell Death Dis. 2023 Sep 1;14(9):581. PMID: 37658049
- 4. Huanhuan Zhu, Xiying Tang, et al. "Exosomal circCLIP1 regulates PM2. 5-induced airway obstruction via targeting SEPT10 in vitro." Ecotoxicol Environ Saf. 2023 Apr 1:254:114750. PMID: 36950992
- 5. Hongli Jiao, Lijie Chen, et al. "IGF2BP3 promotes progression of colorectal cancer and mediates cetuximab resistance by stabilizing EGFR mRNA in an m6A-dependent mechanism." Research Square. rs-1297958/v1.
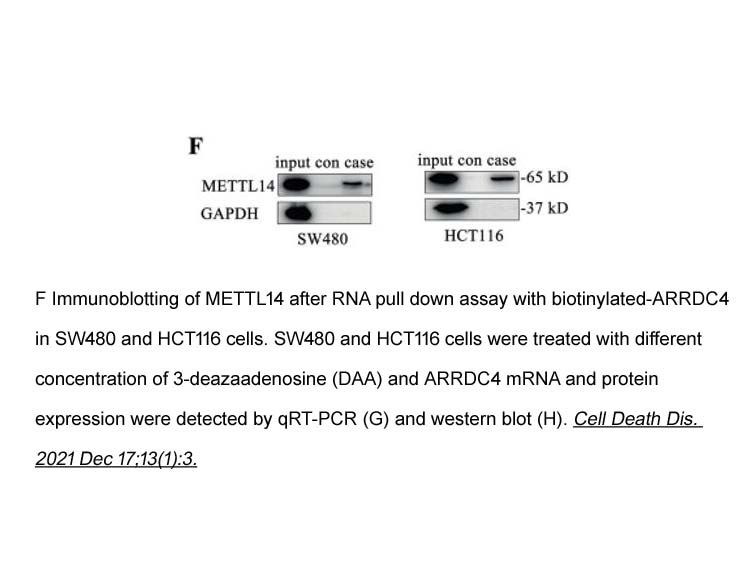
- 6. Hao Wang, Wei Wei, et al. "TCF4 and HuR Mediated-METTL14 Suppresses Dissemination of Colorectal Cancer via N6-Methyladenosine-Dependent Silencing of ARRDC4." Cell Death Dis. 2021 Dec 17;13(1):3. PMID:34916487
- 7. Chen Y, Peng C, et al. "WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1." Mol Cancer. 2019 Aug 22;18(1):127. PMID:31438961
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 266.25 |
| Cas No. | 6736-58-9 |
| Formula | C11H14N4O4 |
| Solubility | ≥26.6 mg/mL in DMSO; insoluble in EtOH; ≥7.53 mg/mL in H2O with gentle warming |
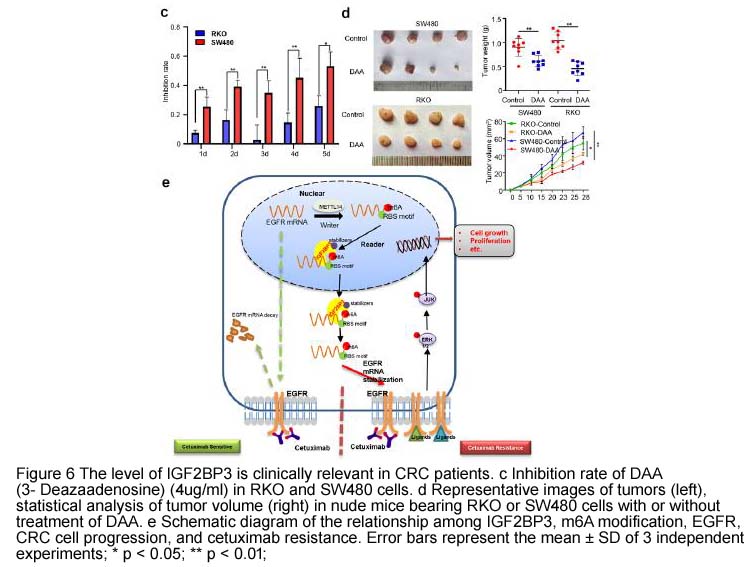
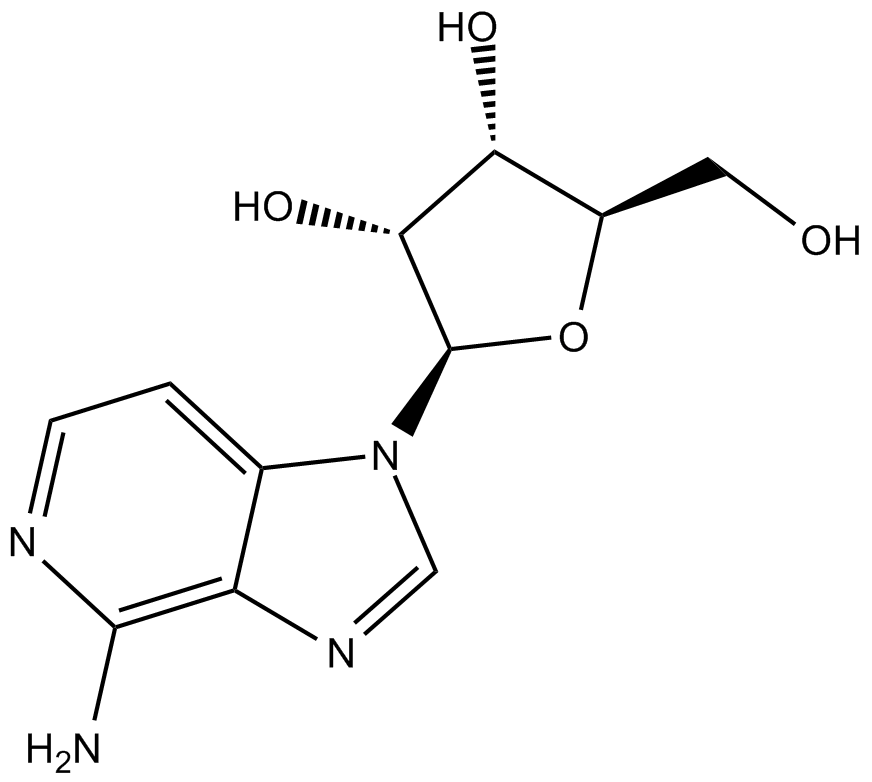
| Chemical Name | (2R,3R,4S,5R)-2-(4-amino-1H-imidazo[4,5-c]pyridin-1-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol |
| SDF | Download SDF |
| Canonical SMILES | Nc1nccc2c1nc[n]2[C@@H]([C@@H]1O)O[C@H](CO)[C@H]1O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data