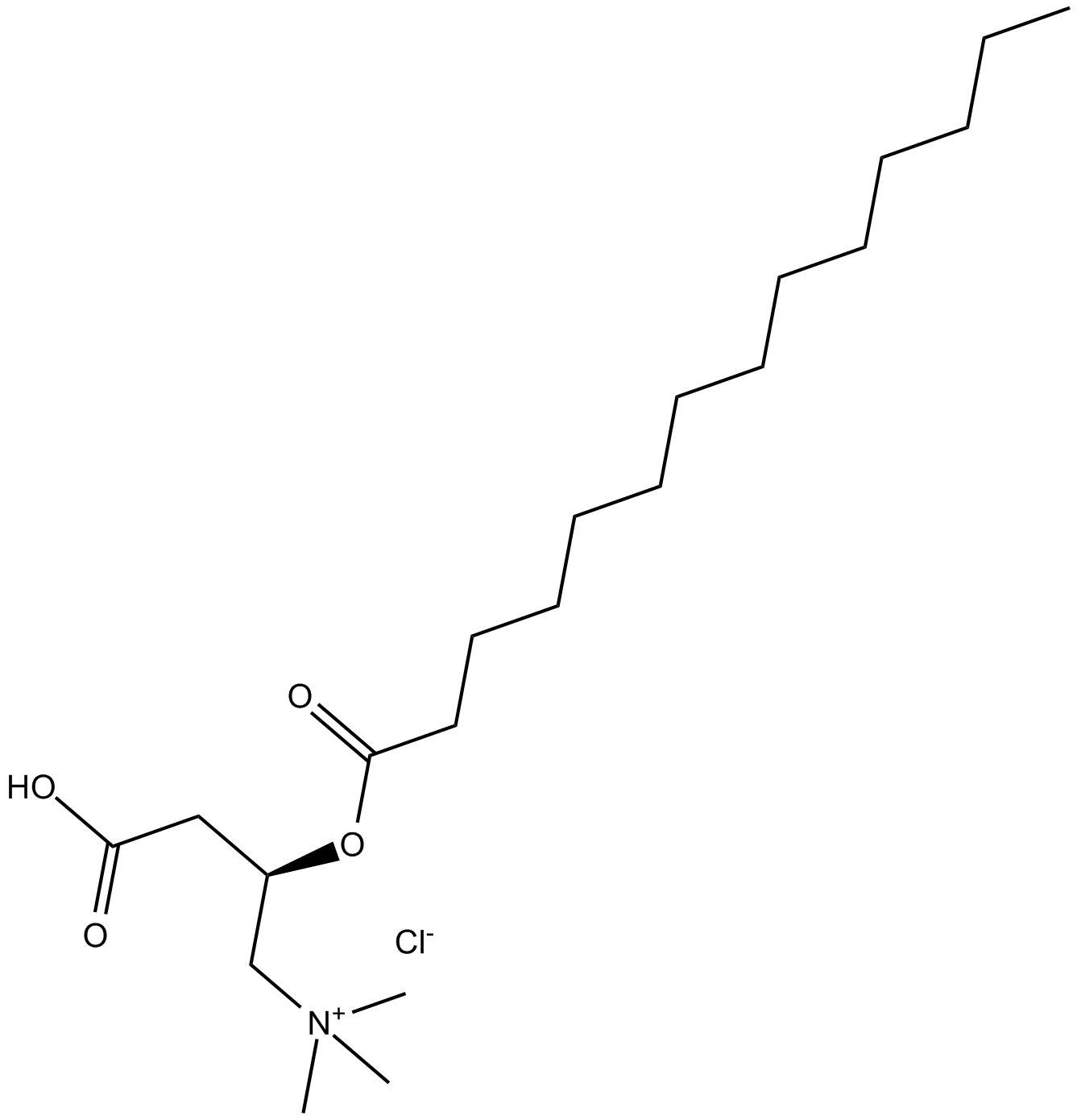
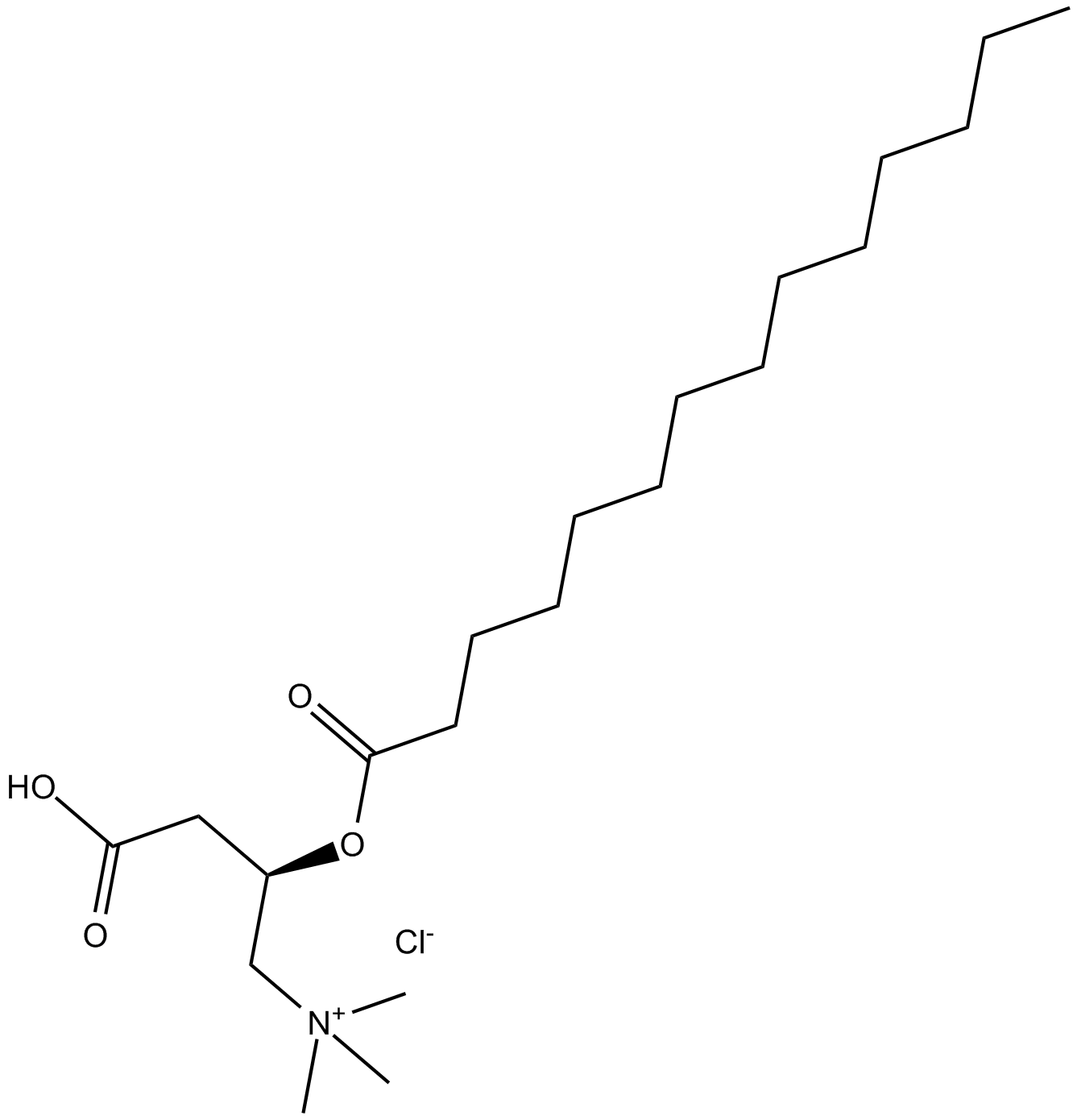
(±)-Myristoylcarnitine chloride
(±)-Myristoylcarnitine chloride is an agonist for cholinergic and a homolog of acetylcarnitine chloride (Cat No. B6273).
Acetylcholine receptor (AChR) is an integral membrane protein receptor for acetylcholine. There are two kinds of AChRs: nicotinic acetylcholine receptors and muscarinic acetylcholine receptors.
(±)-Myristoylcarnitine chloride is a cholinergic agonist and an intermediate in lipid metabolism [1]. In retinal ganglion cells, acetylcarnitine and acetylcholine inhibited GABAergic responses to exogenous GABA and GABAergic inhibitory postsynaptic currents [2].
In dogs with coronary ligation, (-)-carnitine chloride (LCC) (300 mg/kg) and acetyl (-)-carnitine chloride (ALCC) (300 mg/kg) inhibited the ventricular arrhythmia. Also, LCC and ALCC improved oxidative phosphorylation rate and the mitochondrial function [1]. In the mouse hot plate test, acetyl-l-carnitine (ALCAR) (100 mg/kg) exhibited analgesia. While, U-73122 and neomycin (the phospholipase C (PLC) inhibitors) blocked the increase of the pain threshold induced by ALCAR. LiCl that impairing phosphatidylinositol synthesis antagonized the antinociception in a dose-dependent way. PMA and PDBu (PKC activators) blocked the increase of the pain threshold in a dose-dependent way. These results suggested that ALCAR analgesia required the participation of the PLC-IP3 pathway [3].
References:
[1]. Imai S, Matsui K, Nakazawa M, et al. Anti-arrhythmic effects of (-)-carnitine chloride and its acetyl analogue on canine late ventricular arrhythmia induced by ligation of the coronary artery as related to improvement of mitochondrial function. Br J Pharmacol, 1984, 82(2): 533-542.
[2]. B?hring R, Standhardt H, Martelli EA, et al. GABA-activated chloride currents of postnatal mouse retinal ganglion cells are blocked by acetylcholine and acetylcarnitine: how specific are ion channels in immature neurons? Eur J Neurosci, 1994, 6(7): 1089-1099.
[3]. Galeotti N, Bartolini A, Calvani M, et al. Acetyl-L-carnitine requires phospholipase C-IP3 pathway activation to induce antinociception. Neuropharmacology, 2004, 47(2): 286-294.
| Physical Appearance | White solid |
| Storage | Store at -20°C |
| M.Wt | 408.02 |
| Cas No. | 14919-38-1 |
| Formula | C21H42ClNO4 |
| Solubility | <10.2mg/ml in H2O |
| Chemical Name | (R)-3-carboxy-N,N,N-trimethyl-2-(tetradecanoyloxy)propan-1-aminium chloride |
| SDF | Download SDF |
| Canonical SMILES | CCCCCCCCCCCCCC(O[C@H](CC(O)=O)C[N+](C)(C)C)=O.[Cl-] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure