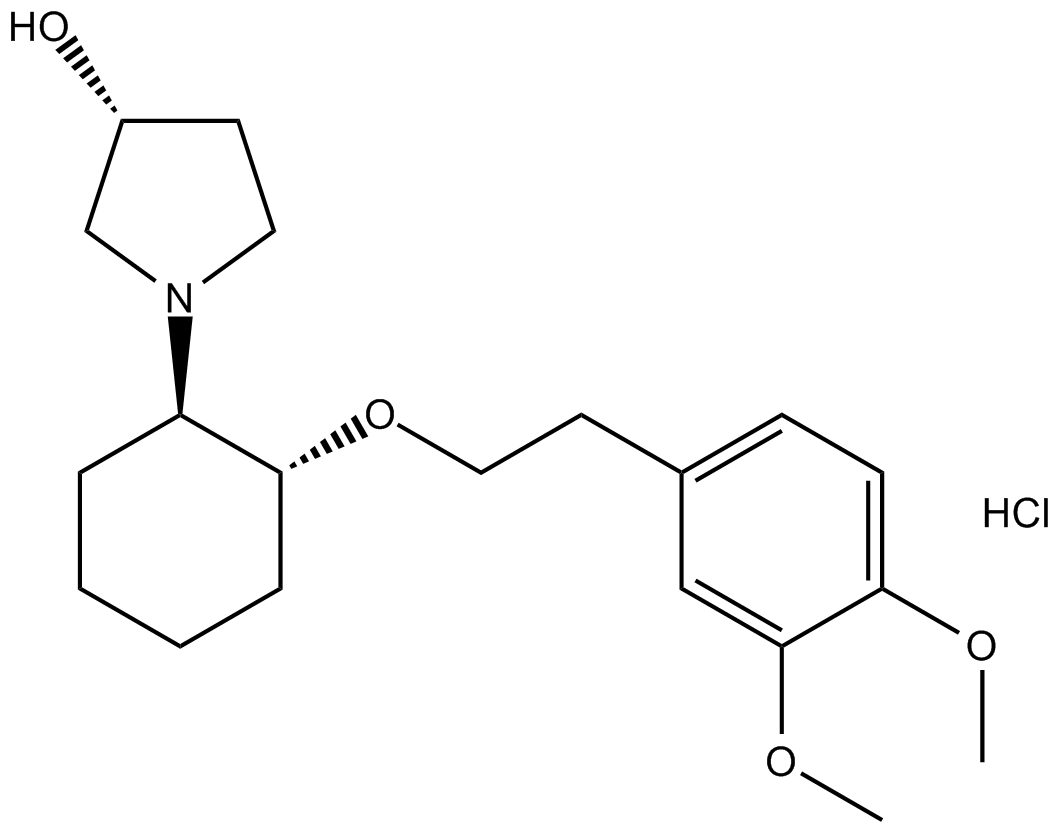
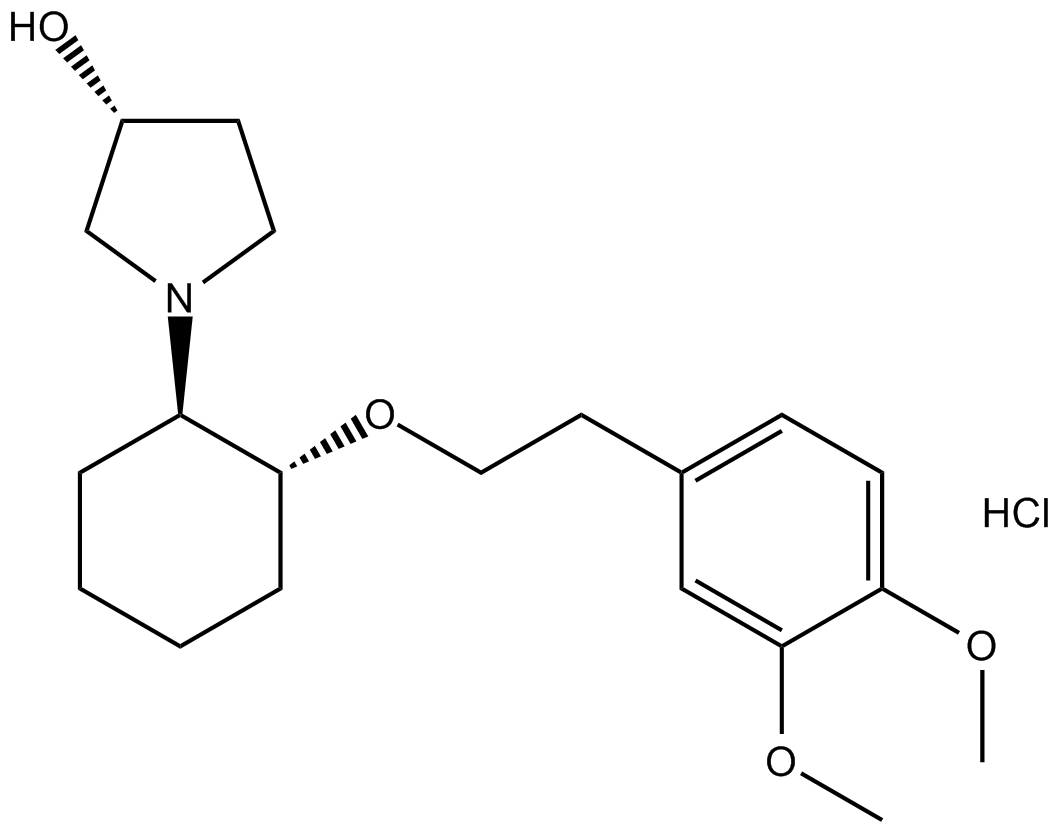
Vernakalant Hydrochloride
Vernakalant Hydrochloride (CAS No. 748810-28-8) is an atrial-selective antiarrhythmic agent whose core bioactivity is the rapid conversion of atrial fibrillation (AF) to sinus rhythm. Its targets include atrial-specific ion channels (IK, Ito, IKr, IKACh), sodium channels (INa, with frequency/voltage/concentration-dependent block), and Kv1.5, Kv4.3, hERG, Nav1.5 channels. It acts by prolonging atrial refractoriness and inhibiting atrial electrical remodeling, with minimal effects on the ventricles. Regarding IC50, the parent drug shows values of 5–45 μM for the above ion channels, while the metabolites RSD1385 and RSD1390 show values of 15–80 μM. It exhibits no significant inhibition of hKCa2.2/2.3 channels (IC50 far above therapeutic concentrations). In PK/PD models, the EC50 related to QTcF is 2276 ng/ml (non-converted AF) and 4222 ng/ml (converted AF), and the EC50 related to systolic blood pressure (SBP) is 1141 ng/ml. Commonly used application concentrations: in vitro cell experiments (HEK293 expressing ion channels) use test concentrations of 0.1–300 μM; in animal experiments (canine AF model) it selectively prolongs atrial refractoriness and terminates AF. Clinical concentrations are based on an intravenous infusion regimen (initial 3 mg/kg, 10-minute infusion; if not converted, an additional 2 mg/kg 10-minute infusion 15 minutes later). A single 3 mg/kg dose yields a peak plasma concentration of about 3.9 μg/ml, and about 4.3 μg/ml after two doses. The effective therapeutic free plasma concentration is 1000–10000 nmol/L. For short-duration AF (3 hours–7 days), the conversion rate reaches 51.7%, with a median conversion time of 8–12 minutes. Tolerability is good; common transient adverse reactions include dysgeusia and sneezing, with no clear risk of torsade de pointes.
References:
[1] Roy D, Pratt CM, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S, Nielsen T, Rasmussen SL, Stiell IG, Coutu B, Ip JH, Pritchett EL, Camm AJ; Atrial Arrhythmia Conversion Trial Investigators. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008 Mar 25;117(12):1518-25. doi: 10.1161/CIRCULATIONAHA.107.723866. Epub 2008 Mar 10. PMID: 18332267.
[2] Mao ZL, Wheeler JJ, Clohs L, Beatch GN, Keirns J. Pharmacokinetics of novel atrial-selective antiarrhythmic agent vernakalant hydrochloride injection (RSD1235): influence of CYP2D6 expression and other factors. J Clin Pharmacol. 2009 Jan;49(1):17-29. doi: 10.1177/0091270008325148. Epub 2008 Oct 16. PMID: 18927241.
[3] Stiell IG, Dickinson G, Butterfield NN, Clement CM, Perry JJ, Vaillancourt C, Calder LA. Vernakalant hydrochloride: A novel atrial-selective agent for the cardioversion of recent-onset atrial fibrillation in the emergency department. Acad Emerg Med. 2010 Nov;17(11):1175-82. doi: 10.1111/j.1553-2712.2010.00915.x. Erratum in: Acad Emerg Med. 2011 Feb;18(2):224. PMID: 21175515.
[4] Pratt CM, Roy D, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S, Retyk E, Drenning DH; Atrial Arrhythmia Conversion Trial (ACT-III) Investigators. Usefulness of vernakalant hydrochloride injection for rapid conversion of atrial fibrillation. Am J Cardiol. 2010 Nov 1;106(9):1277-83. doi: 10.1016/j.amjcard.2010.06.054. PMID: 21029824.
[5] Mao Z, Wheeler JJ, Townsend R, Gao Y, Kshirsagar S, Keirns JJ. Population pharmacokinetic-pharmacodynamic analysis of vernakalant hydrochloride injection (RSD1235) in atrial fibrillation or atrial flutter. J Pharmacokinet Pharmacodyn. 2011 Oct;38(5):541-62. doi: 10.1007/s10928-011-9207-3. Epub 2011 Jul 24. PMID: 21786177.
[6] Simó-Vicens R, Sauter DRP, Grunnet M, Diness JG, Bentzen BH. Effect of antiarrhythmic drugs on small conductance calcium-activated potassium channels. Eur J Pharmacol. 2017 May 15;803:118-123. doi: 10.1016/j.ejphar.2017.03.039. Epub 2017 Mar 18. PMID: 28322838.
| Storage | Store at -20°C |
| M.Wt | 385.93 |
| Cas No. | 748810-28-8 |
| Formula | C20H32ClNO4 |
| Synonyms | RSD1235 |
| Solubility | ≥27.3 mg/mL in DMSO; ≥25.45 mg/mL in EtOH; ≥50.8 mg/mL in H2O |
| Chemical Name | (3R)-1-[(1R,2R)-2-[2-(3,4-dimethoxyphenyl)ethoxy]cyclohexyl]pyrrolidin-3-ol;hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | COc(ccc(CCO[C@H](CCCC1)[C@@H]1N(CC1)C[C@@H]1O)c1)c1OC.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure