THZ531
THZ531 (CAS: 1702809-17-3) is a covalent inhibitor of cyclin-dependent kinase 12 (CDK12) and CDK13, which mediate transcription elongation and RNA processing through phosphorylation of RNA polymerase II (Pol II) in complex with cyclin K. THZ531 irreversibly binds to CDK12 and CDK13, exhibiting inhibitory potency (IC50) of 158 nM and 69 nM, respectively, while showing substantially lower affinity (>50-fold) toward related CDK7 and CDK9. In Jurkat cells, THZ531 induces apoptosis and impairs proliferation (IC50 = 50 nM), reducing Pol II Ser2 phosphorylation and inhibiting transcription of certain super-enhancer-linked and DNA damage response genes. THZ531 serves as a research tool to investigate transcriptional regulation and associated oncogenic pathways.
Reference:
1.Zhang T, Kwiatkowski N, Olson CM, et al. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat Chem Biol. 2016 Oct;12(10):876-84.
- 1. Peuget S, Zhu J, et al. "Thermal proteome profiling identifies oxidative-dependent inhibition of the transcription of major oncogenes as a new therapeutic mechanism for select anticancer compounds." Cancer Res. 2020;canres.2069.2019. PMID:32019870
- 2. Wang C, Vegna S, et al. "Inducing and exploiting vulnerabilities for the treatment of liver cancer." Nature. 2019 Oct 2. PMID:31578521
- 3. Wang C, Wang H, et al. "CDK12 inhibition mediates DNA damage and is synergistic with sorafenib treatment in hepatocellular carcinoma." Gut. 2019 Sep 13. pii: gutjnl-2019-318506. PMID:31519701
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 558.07 |
| Cas No. | 1702809-17-3 |
| Formula | C30H32ClN7O2 |
| Solubility | ≥55.8 mg/mL in DMSO; insoluble in H2O; ≥4.9 mg/mL in EtOH |
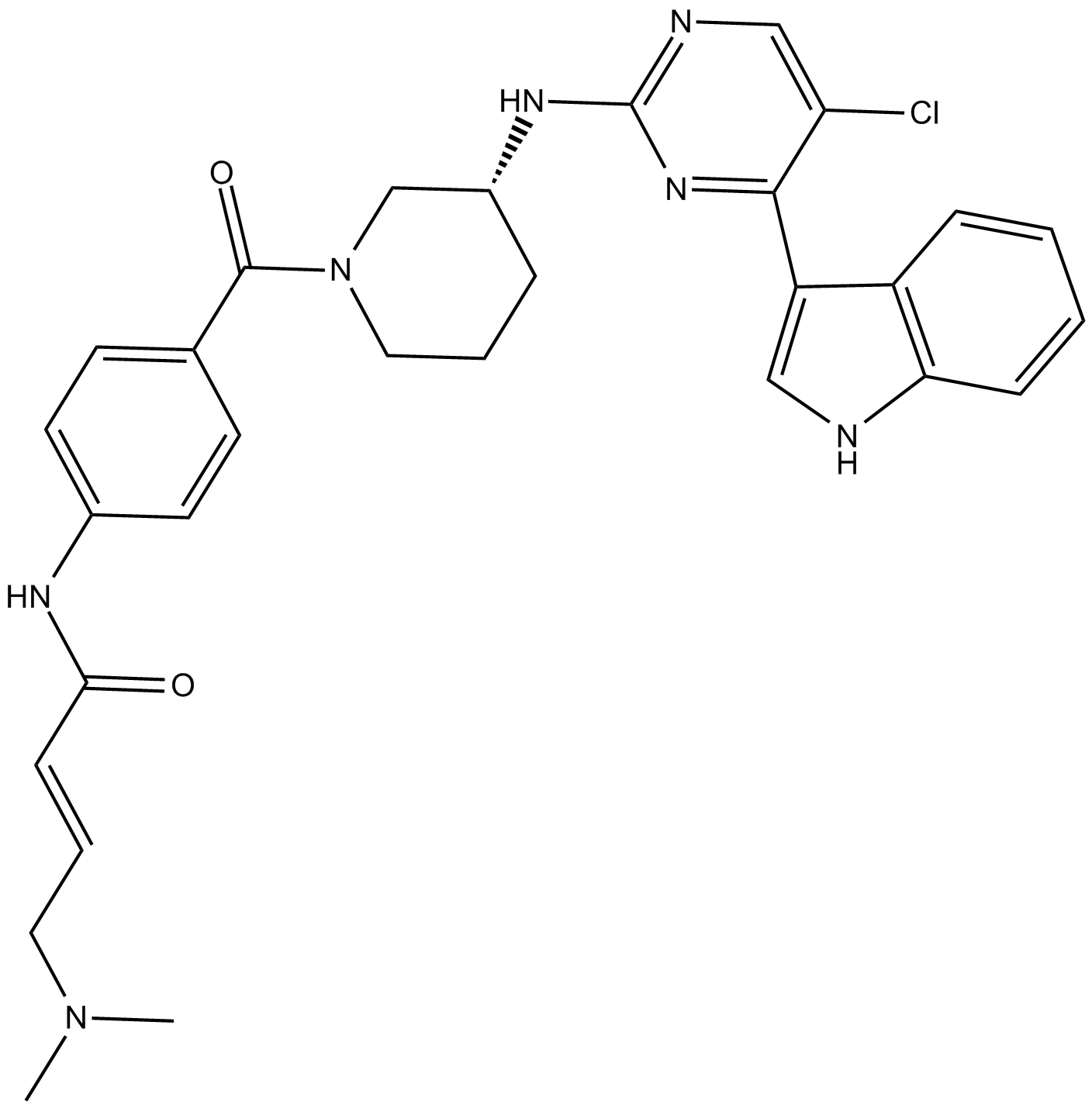
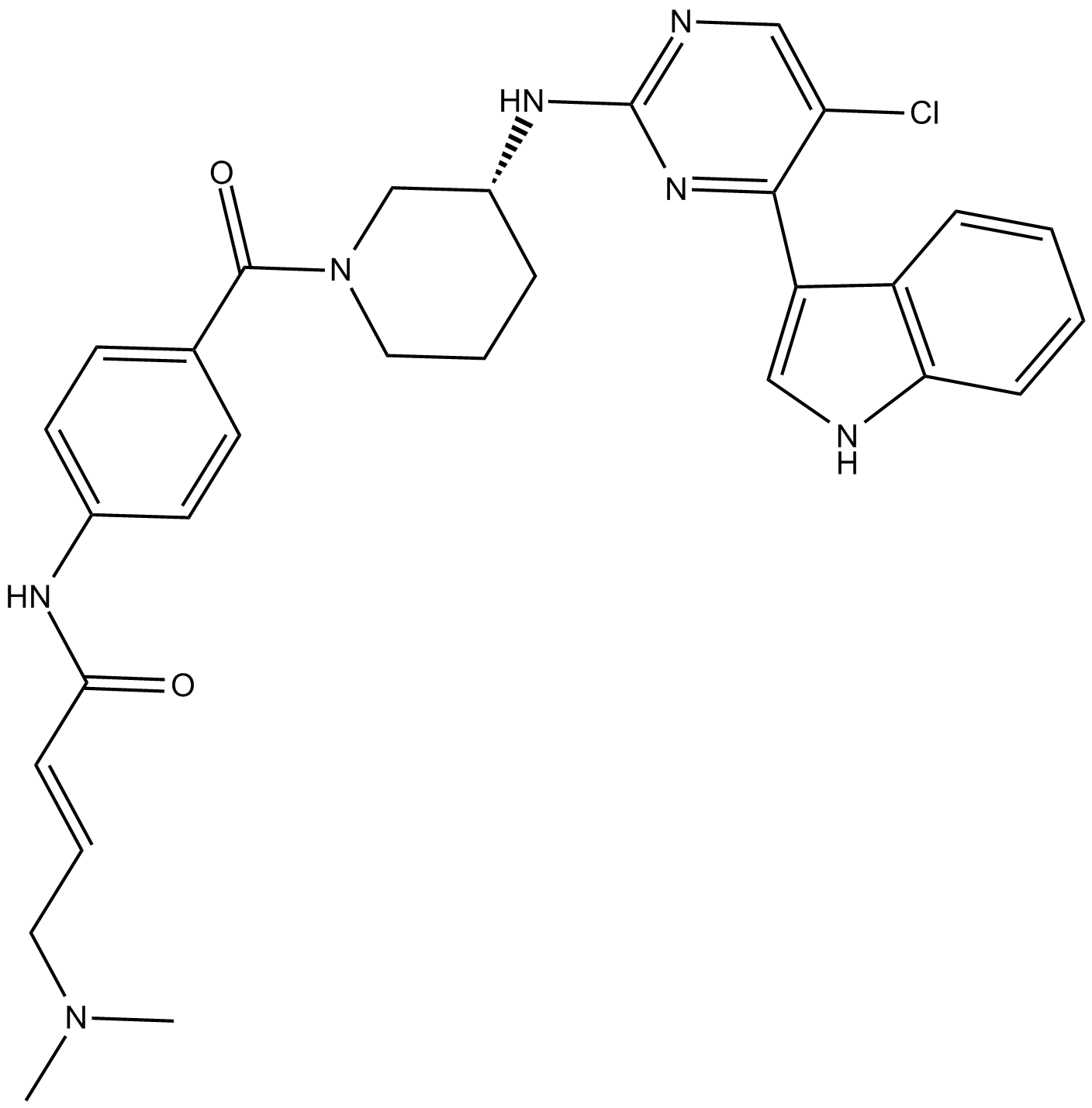
| Chemical Name | (R,E)-N-(4-(3-((5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl)amino)piperidine-1-carbonyl)phenyl)-4-(dimethylamino)but-2-enamide |
| SDF | Download SDF |
| Canonical SMILES | ClC1=CN=C(N[C@H]2CN(C(C3=CC=C(NC(/C=C/CN(C)C)=O)C=C3)=O)CCC2)N=C1C4=CNC5=C4C=CC=C5 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Binding assays |
Radioactive kinase activity measurements were performed at a concentration of 0.2 μM CDK–cyclin complex. Typically, 35 μl reaction volumes of 0.2 μM active kinase were equilibrated in kinase buffer (40 mM Hepes (pH 7.6)), 34 mM NaCl, 34 mM KCl, 10 mM MgCl2, 5% glycerol, and 1× PhosSTOP. Cold ATP to a final concentration of 200 μM and 3 μCi [γ-32P]ATP and 50 μM of a substrate peptide containing five phosphorylation sites were added and the reaction mixture incubated for 30 min at 30 °C at 350 rpm in a thermomixer. Reactions were stopped by adding EDTA to a final concentration of 50 mM. Aliquots of 15 μl each were spotted onto paper squares using the Optitran BA-S85 reinforced membrane. Paper squares were washed three times for 5 min with 0.75% (v/v) phosphoric acid, with at least 5 ml washing solution per paper. Radioactivity was counted in a Beckman Scintillation Counter (Beckman Coulter) for 1 min. Compounds THZ531 was added at varying concentrations to 0.2 μM CDK–cyclin complex and incubated for varying times from 1 to 540 min at 30 °C and 350 rpm before ATP and substrate were added to the reaction mix. |
| Cell experiment [1]: | |
|
Cell lines |
Jurkat cells |
|
Preparation method |
This compound is soluble in DMSO. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
50-1200 nM, 6 h |
|
Applications |
THZ531 treatment led to a dramatic and irreversible decrease in Jurkat cell proliferation with an IC50 of 50 nM. Treatment with escalating doses of THZ531 displayed a dose- and time-dependent increase in the number of cells exhibiting sub-G1 content. Higher doses of THZ531 led to a pronounced annexin V signal, with 30–40% annexin-V-stained cells by 72 h. THZ531 selectively reduced Ser2 phosphorylation levels without appreciable effect on CTD pSer5 or pSer7 levels. Treatment with 50 nM THZ531 resulted in the loss of expression of a small subset of genes. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Zhang T, Kwiatkowski N, Olson C M, et al. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors[J]. Nature chemical biology, 2016. |
|
Quality Control & MSDS
- View current batch:
Chemical structure