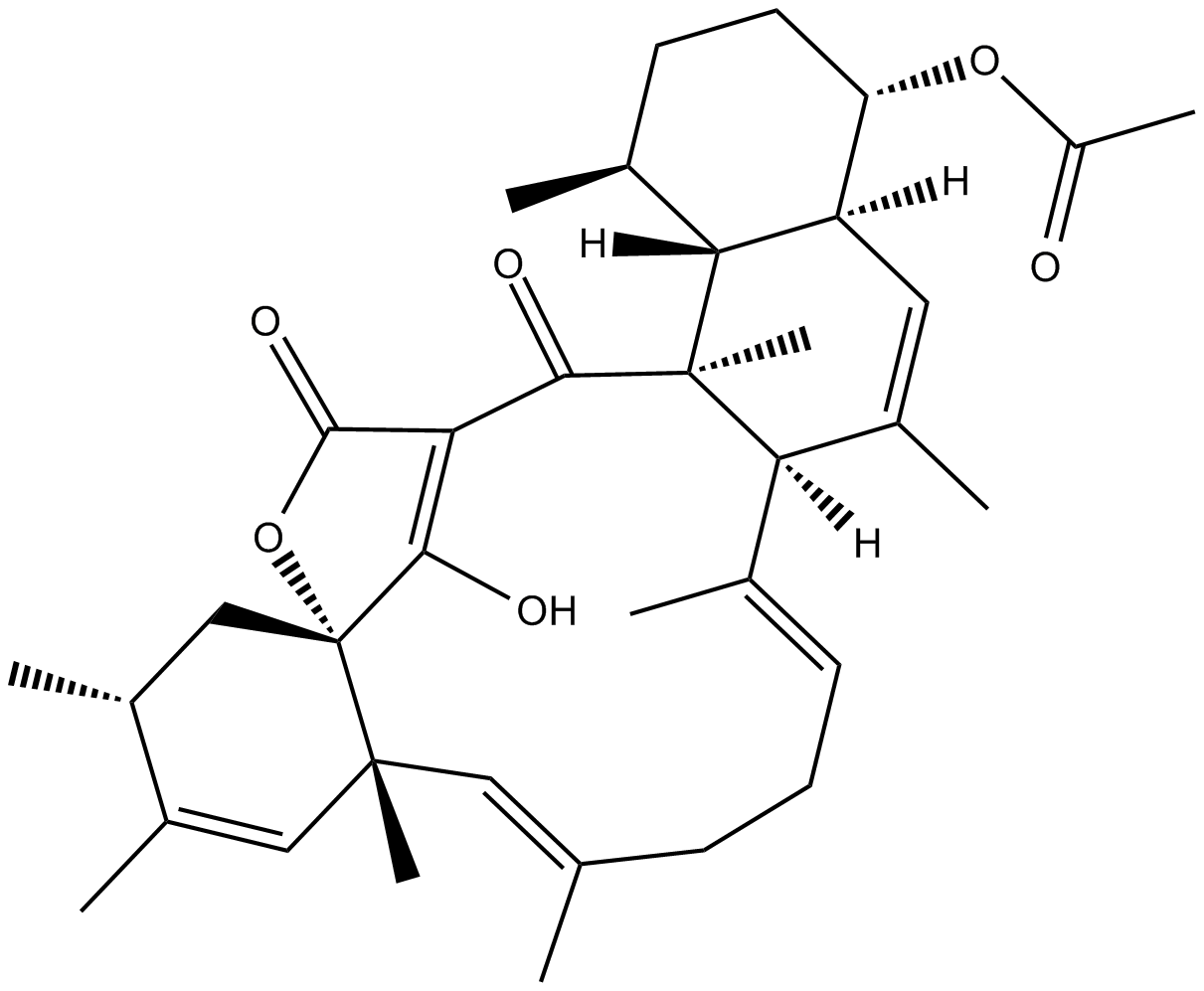
Tetromycin A
Tetromycin A, as an unusual tetronic acid, is a tetronic acid-based antibiotic. It is structurally related to saccharocarcin, chlorothricin, tetrocarcin, kijanimicin and versipelostatin and has been shown to be active against antibiotic resistant and susceptible Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA). MRSA is a strain of bacteria which cannot be killed by a wide range of antibiotics, including methicillin, penicillin, and oxacillin, and causes infections in different parts of the body. The derivatives of tetromycin have been found to inhibit the cysteine protease cathepsin L with Ki values in the low micromolar range and have anti-trypanosomal activity. Tetromycin A probably targets the phosphatidylinositide-3'-kinase/Akt signaling pathway.
Akt, a downstream factor in the phosphatidylinositide-3'-kinase-dependent pathway, mediates a variety of biological responses, including protein synthesis, glucose uptake and the regulation of proliferation and apoptosis, which presumably contributes to acquisition of malignant properties of human cancers [1].
In vitro: Up to now, in vitro study of Tetromycin A is still in the development stage.
In vivo: Up to now, in vivo study of Tetromycin A is still in the development stage.
Reference:
[1]. Nakajima, H., Sakaguchi, K., Fujiwara, I., Mizuta, M., Tsuruga, M., Magae, J., & Mizuta, N. Apoptosis and inactivation of the PI3-kinase pathway by tetrocarcin A in breast cancers. Biochemical and Biophysical Research Communications. 2007; 356(1): 260-265.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 576.8 |
| Cas No. | 180027-83-2 |
| Formula | C36H48O6 |
| Solubility | Soluble in DMSO |
| Chemical Name | (9CI)-(1S,4S,4aS,6aR,7E,11E,12aR,15R,16aS,20aS,20bR)-4-(acetyloxy)-2,3,4,4a,6a,9,10,12a,15,16,20a,20b-dodecahydro-21-hydroxy-1,6,7,11,12a,14,15,20a-octamethyl-18H-16a,19-Metheno-16aH-benzo[b]naphth[2,1-j]oxacyclotetradecin-18,20(1H)-dione |
| SDF | Download SDF |
| Canonical SMILES | CC1=C[C@@]2([H])[C@@]([C@@](C(C3=C4O)=O)(C)[C@]1([H])/C(C)=C/CC/C(C)=C/[C@@]5(C)[C@@]4(OC3=O)C[C@@H](C)C(C)=C5)([H])[C@@H](C)CC[C@@H]2OC(C)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure