Tamsulosin
Tamsulosin (CAS No. 106133-20-4) is a highly selective α₁A-adrenergic receptor blocker. Its core target is the α₁A receptor on the smooth muscles of the bladder neck and prostate, which reduces urethral resistance by relaxing the smooth muscles. The clinically effective therapeutic concentration corresponds to a primary oral dosage of 0.4 mg: for the treatment of ureteral stone expulsion, it is mostly administered as a single dose or short-term course; for the prevention of postoperative urinary retention (POUR), the administration regimens include initiation 12–48 hours before surgery with continuation for 7–14 days postoperatively, or one dose 6 hours before surgery plus another 6–12 hours after surgery, and a low dose of 0.2 mg can be used for adjustment in some scenarios. Its biological activities are mainly reflected in increasing the ureteral stone expulsion rate (80.5% vs. 70.5% in the control group), shortening the stone expulsion time, significantly reducing the risk of postoperative urinary retention (relative risk 0.50) and increasing the maximum urinary flow rate (mean increase of 2.76 mL/sec). It shows more significant stone expulsion effect for stones ≥6 mm. With good safety profile, it only has mild adverse reactions such as retrograde ejaculation and dizziness, and the total incidence rate is not significantly different from that of the control group. It is indicated for the treatment of ureteral stone expulsion and prevention of postoperative urinary retention, especially with better efficacy in patients undergoing anorectal, pelvic, or urogenital surgery and male patients.
References:
[1] Sun Y, Lei GL, Yang L, Wei Q, Wei X. Is tamsulosin effective for the passage of symptomatic ureteral stones: A systematic review and meta-analysis. Medicine (Baltimore). 2019 Mar;98(10):e14796. doi: 10.1097/MD.0000000000014796. PMID: 30855496; PMCID: PMC6417624.
[2] Baysden M, Hein D, Castillo S. Tamsulosin for prevention of postoperative urinary retention: A systematic review and meta-analysis. Am J Health Syst Pharm. 2023 Mar 7;80(6):373-383. doi: 10.1093/ajhp/zxac349. PMID: 36445826.
| Storage | Store at -20°C |
| M.Wt | 408.51 |
| Cas No. | 106133-20-4 |
| Formula | C20H28N2O5S |
| Synonyms | Flomax; Harnalidge |
| Solubility | ≥53.5 mg/mL in DMSO; ≥5.43 mg/mL in EtOH with ultrasonic; insoluble in H2O |
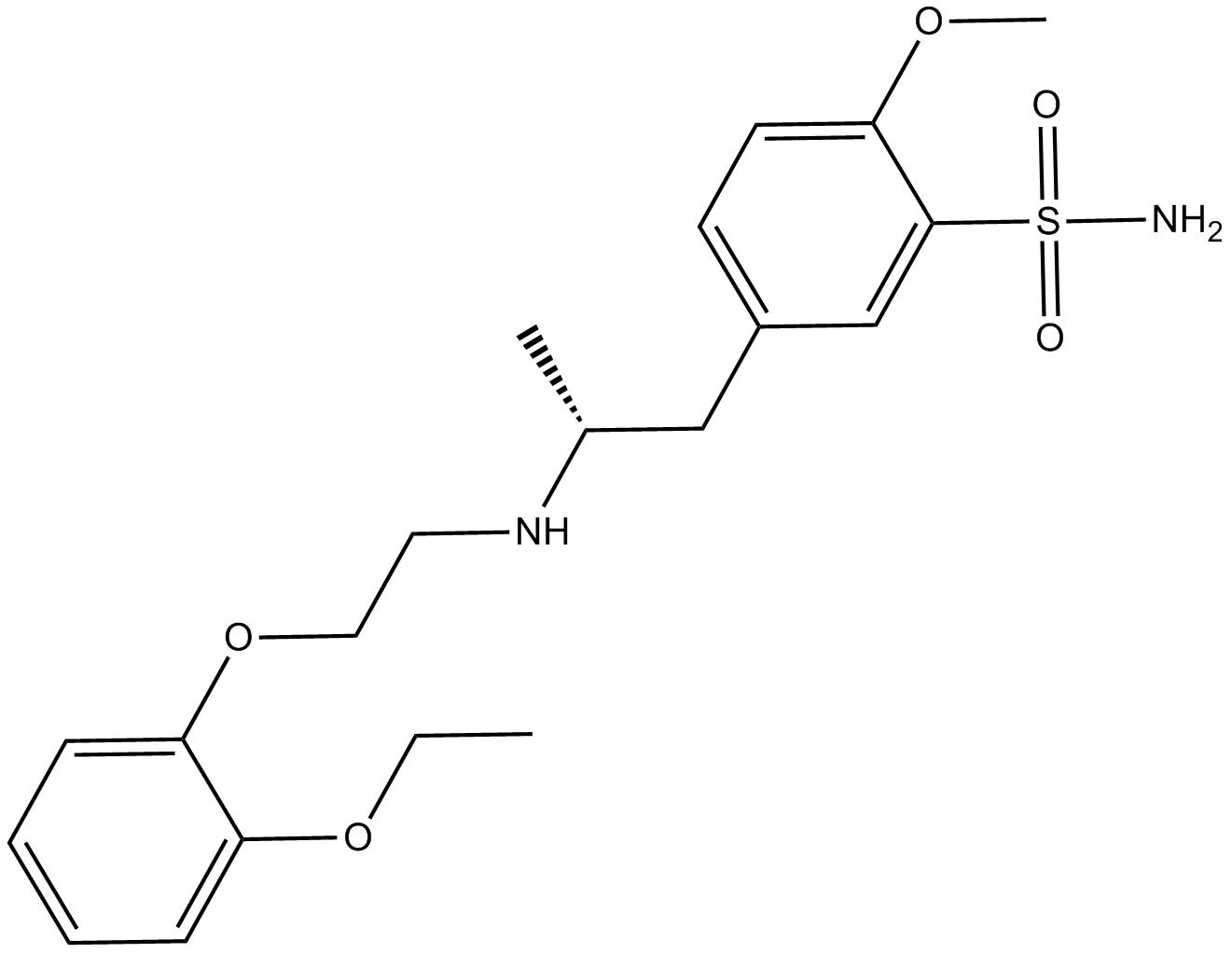
| Chemical Name | (R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-2-methoxybenzenesulfonamide |
| SDF | Download SDF |
| Canonical SMILES | CCOc(cccc1)c1OCCN[C@H](C)Cc(cc1)cc(S(N)(=O)=O)c1OC |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure