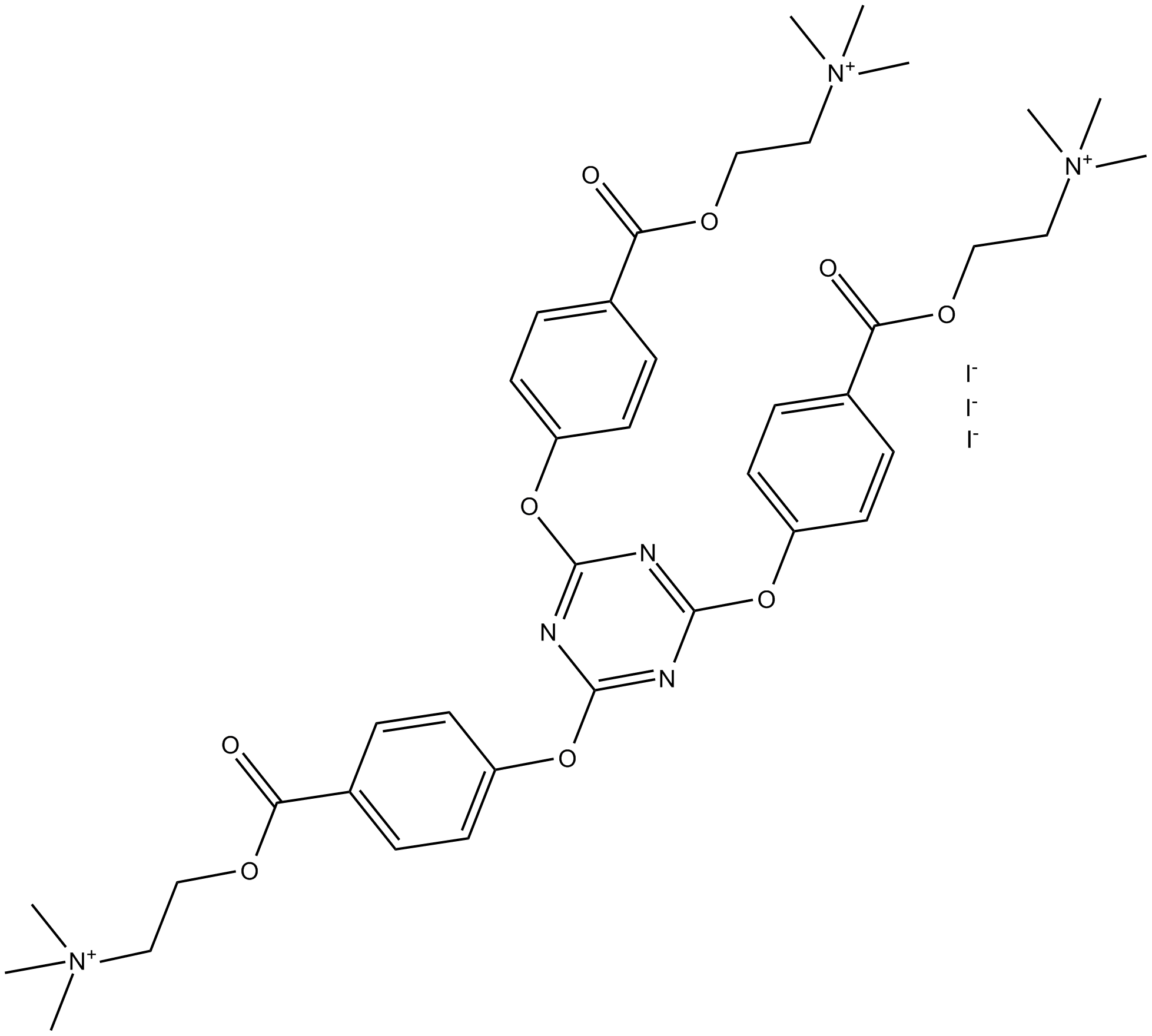
TAE-1
IC50: 0.3 ± 0.02 μM for AChE; 3.9 ± 0.2 μM for BuChE
TAE-1 is an inhibitor of amyloid-β fibril formation and aggregation.
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is characterized by the cerebral accumulation of insoluble aggregates of amyloid-β peptides (Aβ). Although the precise mechanisms governing neuronal loss remains ambiguous, toxicity resulting from Aβ-activated pathways is evident.
In vitro: In a previous study, the authors examined the effects of TAE-1 on differentiated human SH-SY5Y neuronal cells grown in tissue culture. Results showed that the stimulation of neuronal cellular process length and branching was noted. Moreover, the increased synaptophysin suggested that TAE-1 could stimulate synapse formation. Increased expression of MAP2 was also observed, indicating that TAE-1 promoted the differentiation of human neurons. In addition, targeted AChE inhibition was evaluated by electrochemical quantification of the enzymatic product, thiocholine, on unmodified gold screen-printed electrodes. It was found that at increasing TAE-1concentrations, there was a corresponding decrease in the AChE activity resulting in reduced amount of oxidizable thiocholine. The IC50 value was found to be 0.465 μM for TAE-1 [1].
In vivo: Up to now, there is no animla in vivo data reported.
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1] Veloso AJ, Chow AM, Dhar D, Tang DW, Ganesh HV, Mikhaylichenko S, Brown IR, Kerman K. Biological activity of sym-triazines with acetylcholine-like substitutions as multitarget modulators of Alzheimer's disease. ACS Chem Neurosci. 2013 Jun 19;4(6):924-9.
| Storage | Store at 2-8°C |
| M.Wt | 1128.6 |
| Cas No. | 1414469-59-2 |
| Formula | C39H51N6O9·3I |
| Solubility | Soluble in DMSO |
| Chemical Name | 2,2',2'-[1,3,5-triazine-2,4,6-triyltris(oxy-4,1-phenylenecarbonyloxy)]tris[N,N,N-trimethyl-ethanaminium]triiodide |
| SDF | Download SDF |
| Canonical SMILES | O=C(OCC[N+](C)(C)C)C(C=C1)=CC=C1OC2=NC(OC3=CC=C(C(OCC[N+](C)(C)C)=O)C=C3)=NC(OC4=CC=C(C(OCC[N+](C)(C)C)=O)C=C4)=N2.[I-].[I-].[I-] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure