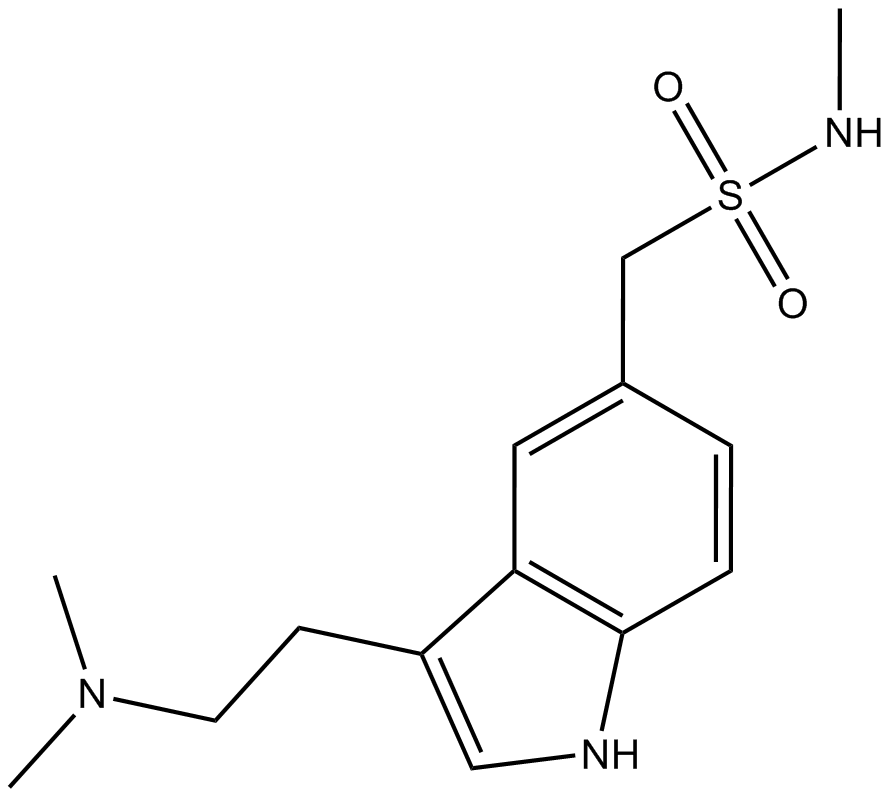
Sumatriptan
Sumatriptan (CAS No. 103628-48-4) is a selective serotonin receptor agonist. Its core targets are the 5-HT₁B (pKi 6.5–8.1), 5-HT₁D (pKi 8.0–8.7), and 5-HT₁F (pIC₅₀ 7.2) receptors. Meanwhile, it is metabolized by monoamine oxidase A (MAO A) and cytochrome P450 enzymes (CYP1A2, CYP2C19, CYP2D6), and can also regulate inflammation-related signaling pathways such as nuclear factor-κB (NF-κB) and nitric oxide synthase (NOS). Its activity is characterized by receptor binding affinity and functional effects.
Commonly used application concentrations are as follows: 10 μM for in vitro enzyme metabolism assays, 10 nM to 10 μM for cellular inflammation models, and a dosage of 0.1–3 mg/kg for animal experiments (administered via intraperitoneal or intravenous injection for inflammation and pain models).
The clinically effective therapeutic concentration corresponds to multiple administration routes: for adult migraine, 100 mg per dose for oral administration and 6 mg per dose for subcutaneous injection; for pediatric emergency cases, intranasal administration is adopted; for cluster headache, 6 mg per dose for subcutaneous injection; for anti-inflammatory treatment, a low dosage of 0.1–1 mg/kg is recommended.
Its biological activities are reflected in constricting cerebral blood vessels and inhibiting the release of calcitonin gene-related peptide (CGRP) to alleviate migraine. At low doses, it exerts anti-inflammatory effects by inhibiting cytokines such as TNF-α and IL-1β, can protect against ischemia/reperfusion injury, and reduce neurogenic inflammation. It has a favorable safety profile, but is contraindicated in patients with cardiovascular diseases. Common mild adverse reactions include gastrointestinal discomfort and dizziness.
References:
[1] Ala M, Ghasemi M, Mohammad Jafari R, Dehpour AR. Beyond its anti-migraine properties, sumatriptan is an anti-inflammatory agent: A systematic review. Drug Dev Res. 2021 Nov;82(7):896-906. doi: 10.1002/ddr.21819. Epub 2021 Apr 1. PMID: 33792938.
[2] Pöstges T, Lehr M. Metabolism of sumatriptan revisited. Pharmacol Res Perspect. 2023 Feb;11(1):e01051. doi: 10.1002/prp2.1051. PMID: 36655303; PMCID: PMC9849828.
[3] Hauser Chatterjee J, Hartford EA, Law E, Barry D, Blume H. Sumatriptan as a First-Line Treatment for Headache in the Pediatric Emergency Department. Pediatr Neurol. 2023 May;142:68-75. doi: 10.1016/j.pediatrneurol.2023.01.016. Epub 2023 Feb 4. PMID: 36958085.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 295.40 |
| Cas No. | 103628-46-2 |
| Formula | C14H21N3O2S |
| Synonyms | GR 43175 free base; Zelrix; Imitrex free base |
| Solubility | ≥14.77 mg/mL in DMSO |
| Chemical Name | 1-(3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)-N-methylmethanesulfonamide |
| SDF | Download SDF |
| Canonical SMILES | CNS(Cc(cc1)cc2c1[nH]cc2CCN(C)C)(=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure