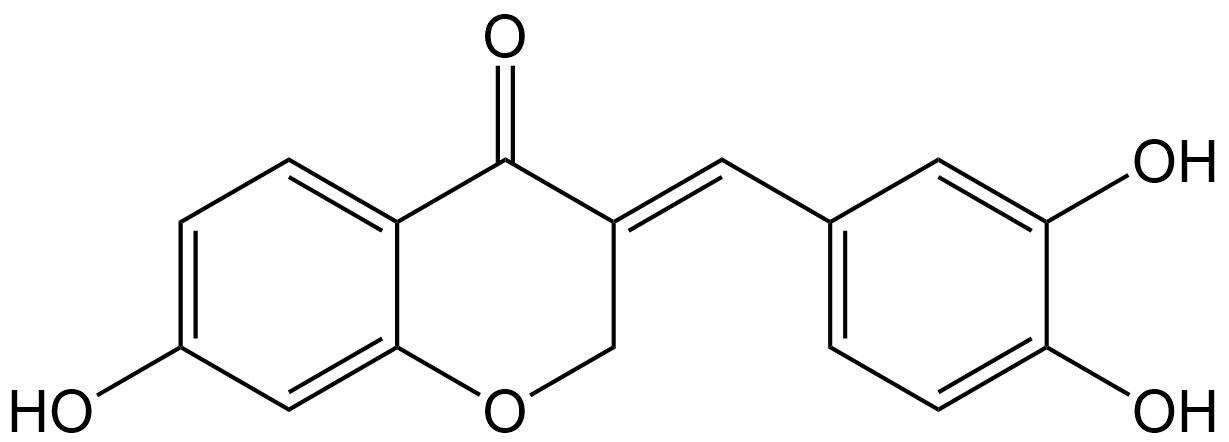
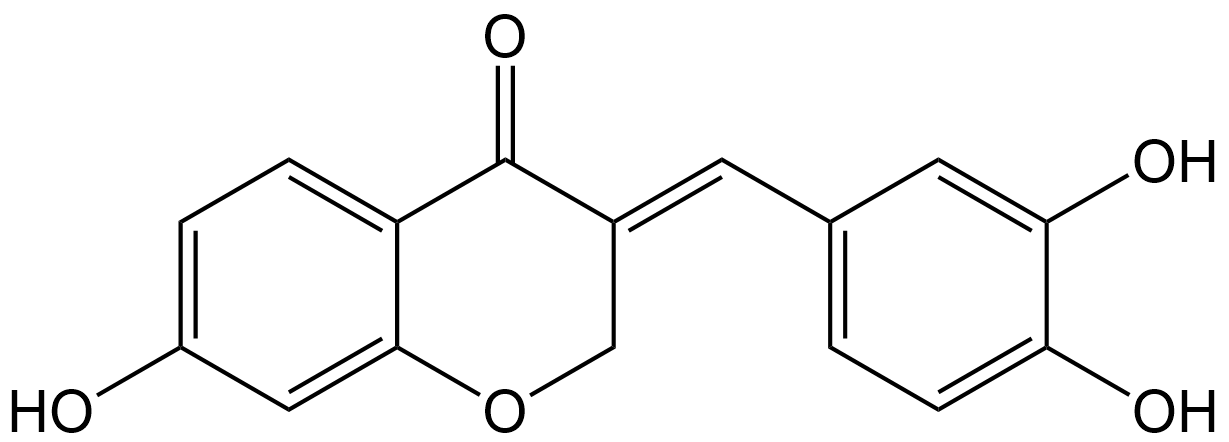
Sappanone A
Sappanone A (CAS 102067-84-5) is a highly isoflavonoid compound with multiple biological activities, which has been isolated from Caesalpinia pulcherrima, Caesalpinia sappan, and campechianum. In vitro experiments show that at a concentration of 10 μM, Sappanone A inhibits the kinase activities of FGFR1, KDR, c-Met, and c-Kit by 76.2%, 59.2%, 37.4%, and 35.4%, respectively. It can also inhibit influenza virus neuraminidase, with IC50 values against H1N1, H3N2, and H9N2 influenza virus NA of 0.7 μM, 1.1 μM, and 1 μM, respectively. This compound exhibits antibacterial activity against Gram-positive bacteria (Bacillus subtilis, Bacillus sphaericus, Staphylococcus aureus) and Gram-negative bacteria (Klebsiella pneumoniae, Chromobacterium violaceum), and can also inhibit the growth of Aspergillus niger and Candida albicans. In addition, Sappanone A can inhibit lipopolysaccharide (LPS)-induced inflammatory responses both in vitro and in vivo, reduce the production of nitric oxide (NO), interleukin-6 (IL-6, Cat# P1023), and prostaglandin E2 (PGE2, Cat# B7005) in RAW264.7 cells, and decrease LPS-induced mortality in mice. In an ovalbumin-induced asthma mouse model, it can also alleviate airway inflammation and mucus hypersecretion by activating the Nrf2 signaling pathway.
References:
[1] Lin LG, Xie H, Li HL, Tong LJ, Tang CP, Ke CQ, Liu QF, Lin LP, Geng MY, Jiang H, Zhao WM, Ding J, Ye Y. Naturally occurring homoisoflavonoids function as potent protein tyrosine kinase inhibitors by c-Src-based high-throughput screening. J Med Chem. 2008 Aug 14;51(15):4419-29. doi: 10.1021/jm701501x. Epub 2008 Jul 9. PMID: 18610999.
[2] Jeong HJ, Kim YM, Kim JH, Kim JY, Park JY, Park SJ, Ryu YB, Lee WS. Homoisoflavonoids from Caesalpinia sappan displaying viral neuraminidases inhibition. Biol Pharm Bull. 2012;35(5):786-90. doi: 10.1248/bpb.35.786. PMID: 22687418.
[3] Das B, Thirupathi P, Ravikanth B, Aravind Kumar R, Sarma AV, Basha SJ. Isolation, synthesis, and bioactivity of homoisoflavonoids from Caesalpinia pulcherrima. Chem Pharm Bull (Tokyo). 2009 Oct;57(10):1139-41. doi: 10.1248/cpb.57.1139. PMID: 19801876.
[4] Lee S, Choi SY, Choo YY, Kim O, Tran PT, Dao CT, Min BS, Lee JH. Sappanone A exhibits anti-inflammatory effects via modulation of Nrf2 and NF-κB. Int Immunopharmacol. 2015 Sep;28(1):328-36. doi: 10.1016/j.intimp.2015.06.015. Epub 2015 Jun 26. PMID: 26122134.
[5] Liu X, Yu D, Wang T. Sappanone A Attenuates Allergic Airway Inflammation in Ovalbumin-Induced Asthma. Int Arch Allergy Immunol. 2016;170(3):180-6. doi: 10.1159/000448331. Epub 2016 Aug 30. PMID: 27576536.
| Storage | Store at -20°C |
| M.Wt | 284.26 |
| Cas No. | 102067-84-5 |
| Formula | C16H12O5 |
| Chemical Name | (E)-3-(3,4-dihydroxybenzylidene)-7-hydroxychroman-4-one |
| SDF | Download SDF |
| Canonical SMILES | OC(C(O)=C1)=CC=C1/C=C2COC3=CC(O)=CC=C3C\2=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure