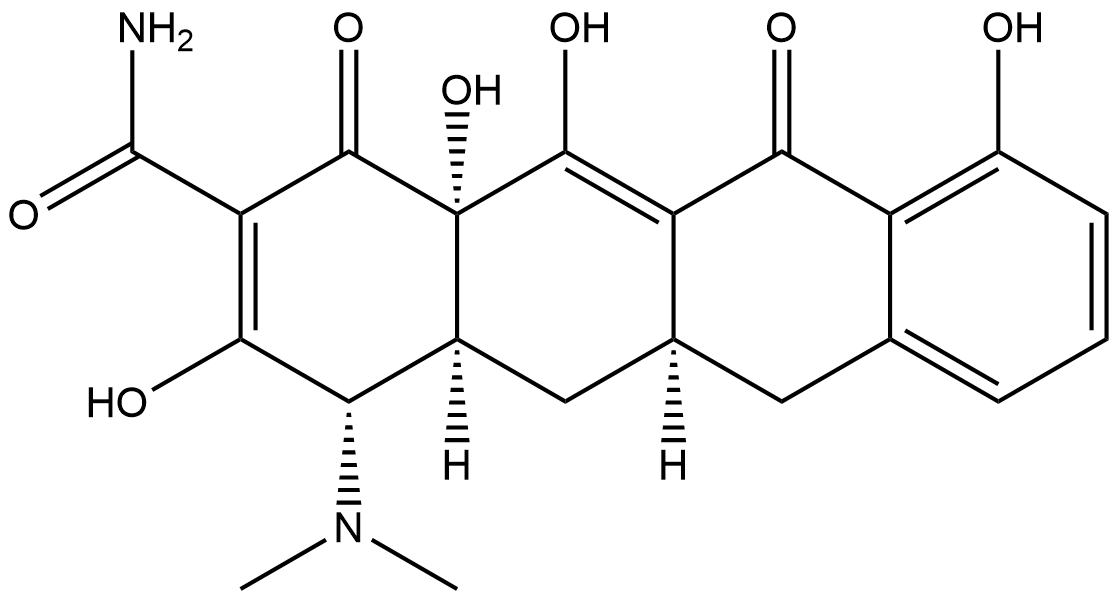
Sancycline
Catalog No.
BA1118
A semi-synthetic tetracycline produced by hydrothermalysis of the chlorine and benzyl hydroxyl groups of Declomycin.
Featured Products
Sancycline (CAS No.: 808-26-4) is a rare semisynthetic tetracycline antibiotic obtained by hydrogenating the chloro and phenyl hydroxyl moieties of Declomycin. As the structurally simplest representative among early tetracyclines, Sancycline was the first tetracycline compound to be fully synthesized by Conover and colleagues. Its primary mechanism of action is to reversibly bind to the bacterial 30S ribosomal subunit, blocking the entry of aminoacyl-tRNA into the ribosomal A site, thereby inhibiting protein synthesis and exhibiting broad-spectrum antibacterial activity. In vitro studies show that Sancycline inhibits a variety of Gram-positive and Gram-negative bacteria, with activity typically in the low micromolar range. Its use in cellular and animal models is diverse, and the specific dose and concentration depend on the experimental design and research objectives.
| Physical Appearance | A solid |
| Storage | -20°C |
| M.Wt | 414.41 |
| Cas No. | 808-26-4 |
| Formula | C21H22N2O7 |
| Synonyms | Bonomycin;6-Demethyl-6-deoxytetracycline |
| Solubility | ≥12.9 mg/mL in DMSO with ultrasonic; insoluble in EtOH; insoluble in H2O |
| Chemical Name | (4S,4aS,5aR,12aS)-4-(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide |
| SDF | Download SDF |
| Canonical SMILES | O=C(N)C1=C([C@@H](N(C)C)[C@@](C[C@@](CC2=CC=CC(O)=C2C3=O)([H])C3=C4O)([H])[C@@]4(O)C1=O)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |