PPT
PPT, abbreviated from propyl pyrazole triol, is a potent, selective agonist of estrogen receptor α (ERα), with a reported 410-fold selectivity for ERα over ERβ. ERα is one of two main types of estrogen receptor, a nuclear receptor that is activated by the sex hormone estrogen and involved in normal developmental, physiological, and reproductive processes in vertebrates. Compared with ERβ, ERα is encoded by a distinct gene, and differs in its relative and absolute tissue distribution. Since PPT exhibits subtype-selective property for estrogen receptors, it may serve as a useful tool for exploring how estrogens work through different ER subtypes.
References:
1. Sotoca AM, van den Berg H, Vervoort J, et al. Influence of cellular ERα/ERβ ratio on the ERα-agonist induced proliferation of human T47D breast cancer cells. Toxicological Sciences, 2008, 105(2): 303-311.
2. Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. Journal of Medicinal Chemistry, 2000, 43(26): 4934-4947.
3. Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology, 2002, 143(11): 4172-4177.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 386.45 |
| Cas No. | 263717-53-9 |
| Formula | C24H22N2O3 |
| Solubility | ≥95.4 mg/mL in DMSO,≥48.9 mg/mL in EtOH,insoluble in H2O |
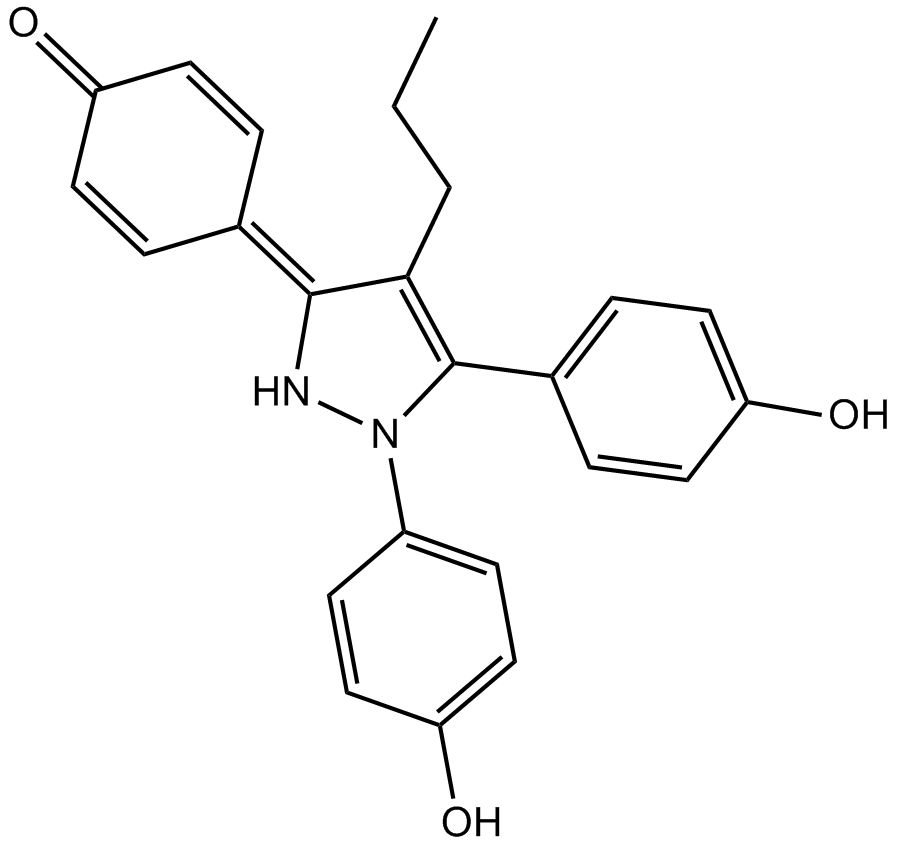
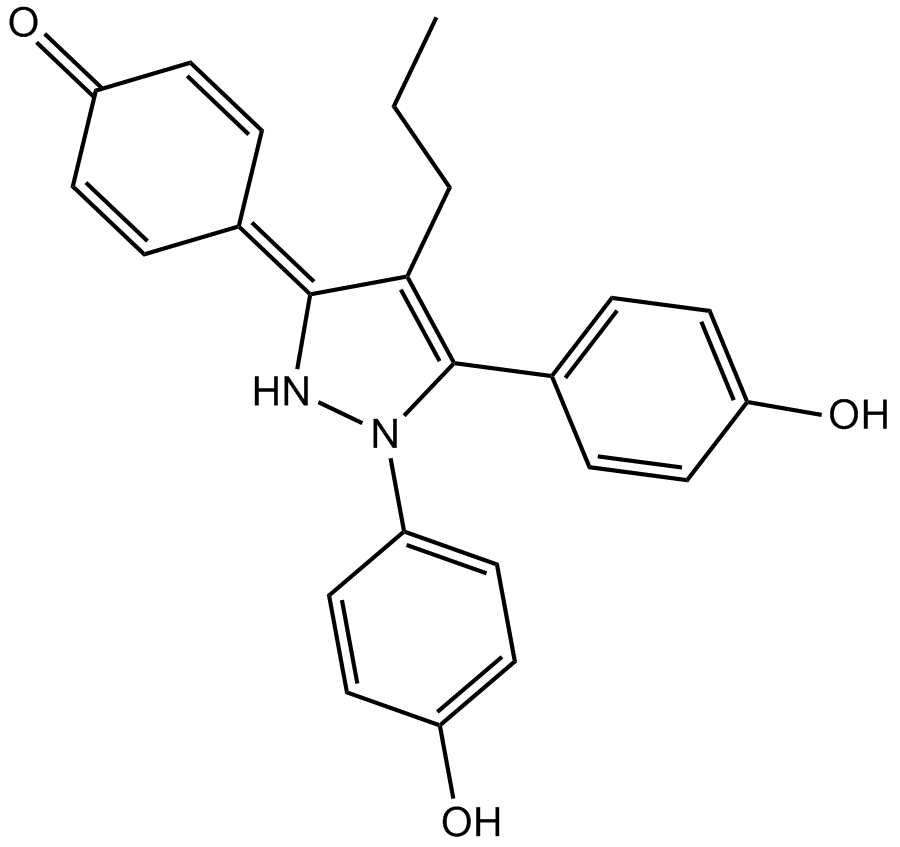
| Chemical Name | 4-(1,5-bis(4-hydroxyphenyl)-4-propyl-1H-pyrazol-3(2H)-ylidene)cyclohexa-2,5-dienone |
| SDF | Download SDF |
| Canonical SMILES | CCCC1=C(c(cc2)ccc2O)N(c(cc2)ccc2O)NC1=C(C=C1)C=CC1=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[3] | |
|
Cell lines |
Saos-2 cells expressing either human ERα or ERβ |
|
Reaction Conditions |
1 μM PPT for 24 h incubation |
|
Applications |
PPT did not up-regulate metallothionein-II mRNA which is regulated only by ERβ, and only up-regulated IGFBP-4 mRNA (regulated by both ERα and ERβ) in Saos-2 cells expressing ERα. |
| Animal experiment:[3] | |
|
Animal models |
Sexually immature Sprague Dawley rats |
|
Dosage form |
5 ~ 1000 μg/rat Once daily by subcutaneous injection for 3 days |
|
Applications |
In a short-term (4 d) uterotrophic assay, PPT was found to be as efficacious as 17α-ethinyl-17β-estradiol in stimulating uterine weight gain and up-regulating complement 3 gene expression. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Sotoca AM, van den Berg H, Vervoort J, et al. Influence of cellular ERα/ERβ ratio on the ERα-agonist induced proliferation of human T47D breast cancer cells. Toxicological Sciences, 2008, 105(2): 303-311. 2. Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. Journal of Medicinal Chemistry, 2000, 43(26): 4934-4947. 3. Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology, 2002, 143(11): 4172-4177. |
|
Quality Control & MSDS
- View current batch:
Chemical structure