Pareruptorin A
Praeruptorin A (CAS No.: 73069-27-9) is an angular pyranocoumarin compound derived from Peucedanum praeruptorum Dunn. Its core therapeutic targets include DMT1, STAT-1/3, NF-κB, ERK1/2, and MMP1, and it can also bind to molecules such as IL-1β, HMOX1, PTGS2, and Abca1. The effective in vitro concentration varies by cell type: 1~5 μM in RAW264.7 macrophages (no cytotoxicity, 5 μM being optimal), 10~30 μM in human hepatocellular carcinoma cells (SK-Hep-1, PLC/PRF/5, Huh-7), 0.4~0.6 μM in AC16 human cardiomyocytes, 1 μM in H9C2 rat cardiomyocytes, and 75 μg/mL in Caco-2 colonic epithelial cells. The in vivo therapeutic doses are 0.8~1.2 mg/kg/d via intraperitoneal injection in mice (to alleviate doxorubicin-induced cardiomyopathy) and 30 mg/kg/d via intragastric administration (to alleviate ulcerative colitis). Its biological activities are multifaceted: it inhibits ferroptosis by suppressing DMT1-mediated Fe²⁺ overload, alleviates doxorubicin-induced myocardial injury, and synergistically enhances the antitumor activity of doxorubicin; it downregulates pro-inflammatory factors (TNF-α, IL-6, IL-1β) and upregulates anti-inflammatory factors (IL-10, TGF-β) by inhibiting the phosphorylation of STAT-1/3 and the activation of AKT, p65, and p38, inhibits colonic cell apoptosis, repairs the intestinal barrier (ZO-1, occludin, claudin-1), and alleviates ulcerative colitis; it exerts anti-inflammatory effects by inhibiting the activation of the NF-κB pathway and reducing the expression of inflammatory factors, PTGS2, and HMOX1; it downregulates MMP1 by activating the ERK1/2 signaling pathway, thereby inhibiting the migration and invasion of hepatocellular carcinoma cells. Additionally, it exhibits no significant cytotoxicity or multi-organ damage within the effective dose range, showing good safety.
References:
[1] Hu Y, Zhang H, Liang W, Xu P, Lou K, Pu J. Rapid and Simultaneous Measurement of Praeruptorin A, Praeruptorin B, Praeruptorin E, and Moisture Contents in Peucedani Radix Using Near-Infrared Spectroscopy and Chemometrics. J AOAC Int. 2020 Apr 1;103(2):504-512. doi: 10.5740/jaoacint.19-0126. PMID: 31561752.
[2] Yu CL, Yu YL, Yang SF, Hsu CE, Lin CL, Hsieh YH, Chiou HL. Praeruptorin A reduces metastasis of human hepatocellular carcinoma cells by targeting ERK/MMP1 signaling pathway. Environ Toxicol. 2021 Apr;36(4):540-549. doi: 10.1002/tox.23059. Epub 2020 Nov 23. PMID: 33226171.
[3] Hu J, Liu R, Yang Z, Pan X, Li Y, Gong Y, Guo D. Praeruptorin A inhibits the activation of NF-κB pathway and the expressions of inflammatory factors in poly (I:C)-induced RAW264.7 cells. Chem Biol Drug Des. 2023 Nov;102(5):1110-1120. doi: 10.1111/cbdd.14310. Epub 2023 Jul 27. PMID: 37500542.
[4] Li D, Chen Y, Zhang B, Heng X, Yin J, Zhao P, Sun N, Shao C. Praeruptorin A screened by a ferrous ion probe inhibited DMT1 and ferroptosis to attenuate Doxorubicin-induced cardiomyopathy. Eur J Med Chem. 2025 Feb 5;283:117108. doi: 10.1016/j.ejmech.2024.117108. Epub 2024 Nov 26. PMID: 39615370.
| Storage | Store at 4°C away from light. |
| M.Wt | 386.40 |
| Cas No. | 73069-27-9 |
| Formula | C21H22O7 |
| Solubility | ≥50.8 mg/mL in DMSO; ≥12.68 mg/mL in EtOH with ultrasonic; insoluble in H2O |
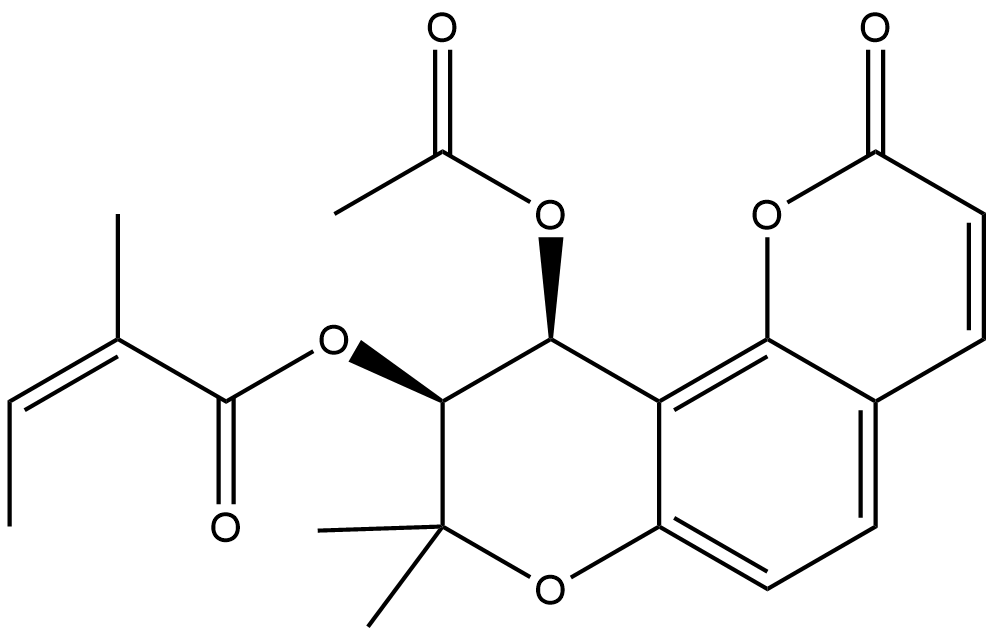
| Chemical Name | (9S,10S)-10-acetoxy-8,8-dimethyl-2-oxo-9,10-dihydro-2H,8H-pyrano[2,3-f]chromen-9-yl (Z)-2-methylbut-2-enoate |
| SDF | Download SDF |
| Canonical SMILES | O=C(/C(C)=C\C)O[C@H]1[C@@H](OC(C)=O)C2=C(C(C=C3)=CC=C2OC1(C)C)OC3=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |