Oteseconazole
Oteseconazole (CAS No. 1340593-59-0) is a potent and selective tetrazole CYP51 (lanosterol 14α-demethylase) inhibitor, whose core bioactivity is to inhibit the growth of Candida and other fungi. By targeting and binding fungal CYP51, it blocks ergosterol biosynthesis and disrupts cell membrane integrity, and it shows high selectivity for human cytochrome P450 enzymes, reducing the risk of drug-drug interactions. IC?? data are well defined: the IC?? for human CYP3A4 is 65 μM, which is significantly higher than that of imidazole and triazole drugs. Its MIC is pathogen-specific: the MIC?? values against Candida albicans (SC5314, CPCC400616), Candida tropicalis, Candida parapsilosis, Candida glabrata, Candida krusei, Cryptococcus neoformans, etc. are all ≤0.00625~0.1 μg/mL, it shows no inhibitory activity against Aspergillus fumigatus (MIC>64 μg/mL), and it remains active against fluconazole-resistant Candida. Common application concentrations: in vitro antimicrobial tests use gradient concentrations of 0.00625~0.1 μg/mL (for Candida spp.). Clinically effective therapeutic concentrations correspond to the approved dosing for its indication, for the prevention of recurrent vulvovaginal candidiasis (RVVC), maintaining plasma concentrations above the MIC values for Candida via oral administration. The high selectivity achieved through structural optimization significantly reduces the risk of drug-drug interactions in clinical use and confers good tolerability.
References:
[1] Yu J, Wang Y, Ragueneau-Majlessi I. Risk of Enzyme- and Transporter-mediated Drug Interactions With Drugs Approved by the US Food and Drug Administration in 2022: A Detailed Analysis of In Vitro and Clinical Data Available in New Drug Application Reviews. Clin Ther. 2024 Jun;46(6):499-508. doi: 10.1016/j.clinthera.2024.04.008. Epub 2024 May 10. PMID: 38734524.
[2] Sivasankar S, Boppe A, Grobusch MP, Jeyaraj S. Evaluation of MMV Pandemic Response Box compounds to identify potent compounds against clinically relevant bacterial and fungal clinical isolates in vitro. New Microbes New Infect. 2024 Jun 20;60-61:101444. doi: 10.1016/j.nmni.2024.101444. PMID: 39040124; PMCID: PMC11261442.
[3] Sun Y, Luo Z, Li K, Wang H, Gao Z, Zhang J, Liu R, Liu R, Wu X, Liu N, Zhang H, Su X, Yin W, Zhao D, Cheng M. Design, Synthesis, and Biological Activity Evaluation of Deuterated Diphenyl Azole Alcohol-Based CYP51 Inhibitors. J Med Chem. 2025 Jun 12;68(11):12229-12257. doi: 10.1021/acs.jmedchem.5c01068. Epub 2025 Jun 3. PMID: 40461049.
[4] Luo Z, Liu R, Sun Y, Zhao L, Zhang J, Li K, Gao Z, Liu N, Zhang H, Wu X, Liu J, Hao W, Su X, Zhao D, Cheng M. Discovery of a potent, broad-spectrum and in vivo effective deuterated tetrazole CYP51 inhibitor. Eur J Med Chem. 2025 Oct 15;296:117817. doi: 10.1016/j.ejmech.2025.117817. Epub 2025 May 28. PMID: 40472486.
| Physical Appearance | A solid |
| Storage | -20°C |
| M.Wt | 527.39 |
| Cas No. | 1340593-59-0 |
| Formula | C23H16F7N5O2 |
| Synonyms | VT-1161 |
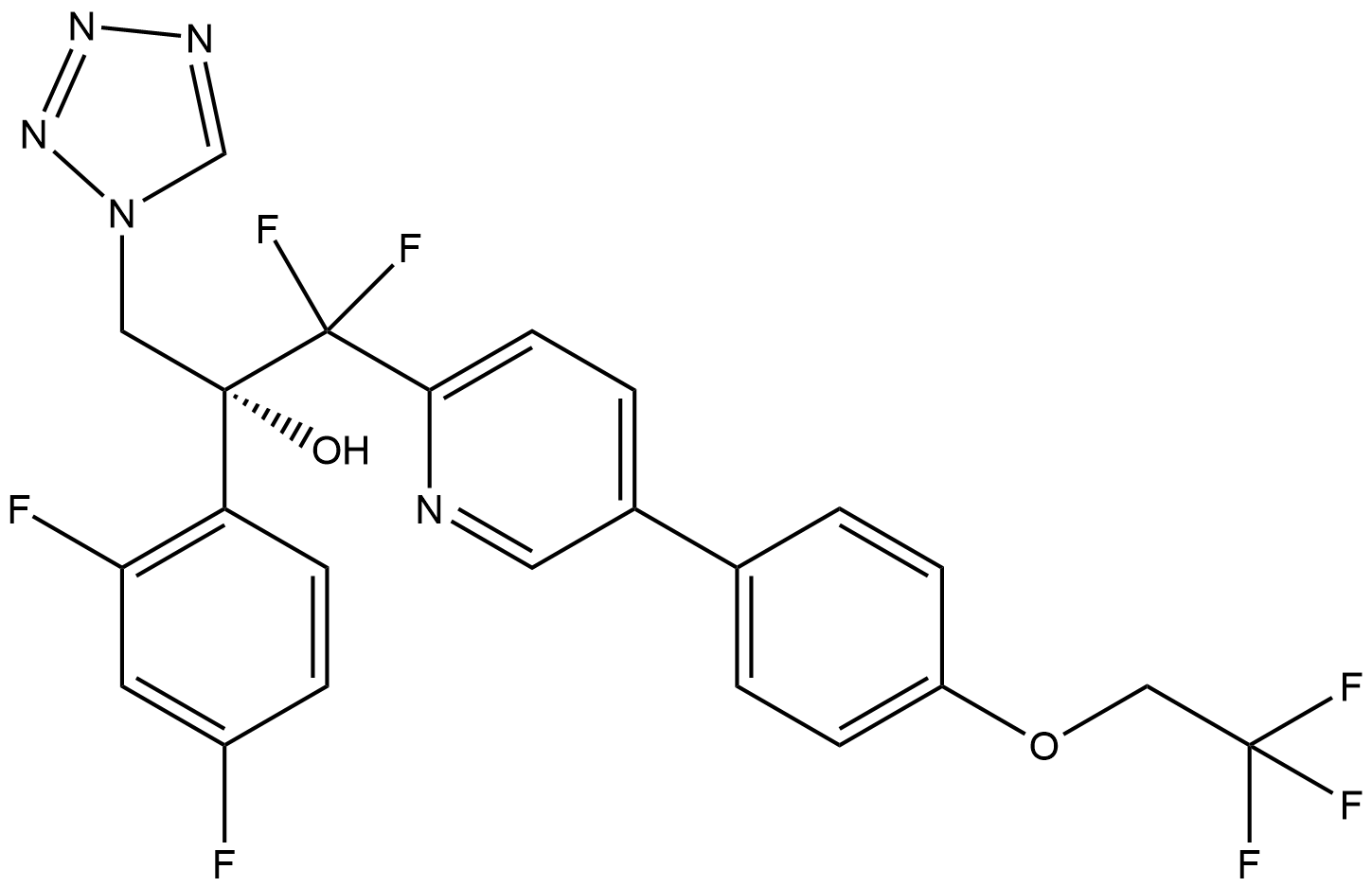
| Chemical Name | (R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-(5-(4-(2,2,2-trifluoroethoxy)phenyl)pyridin-2-yl)propan-2-ol |
| SDF | Download SDF |
| Canonical SMILES | FC1=C(C=CC(F)=C1)[C@](CN2C=NN=N2)(O)C(F)(F)C3=CC=C(C4=CC=C(OCC(F)(F)F)C=C4)C=N3 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |