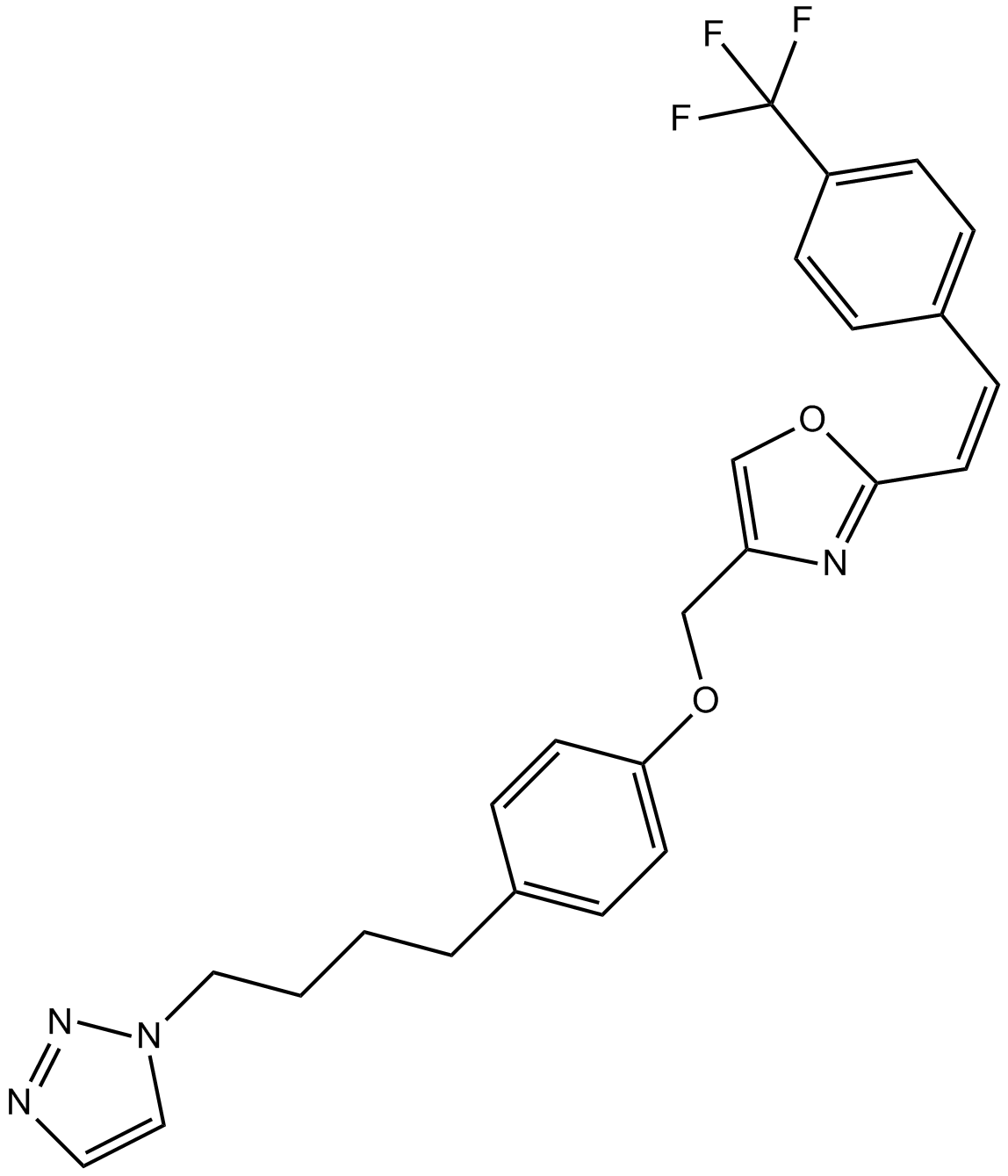
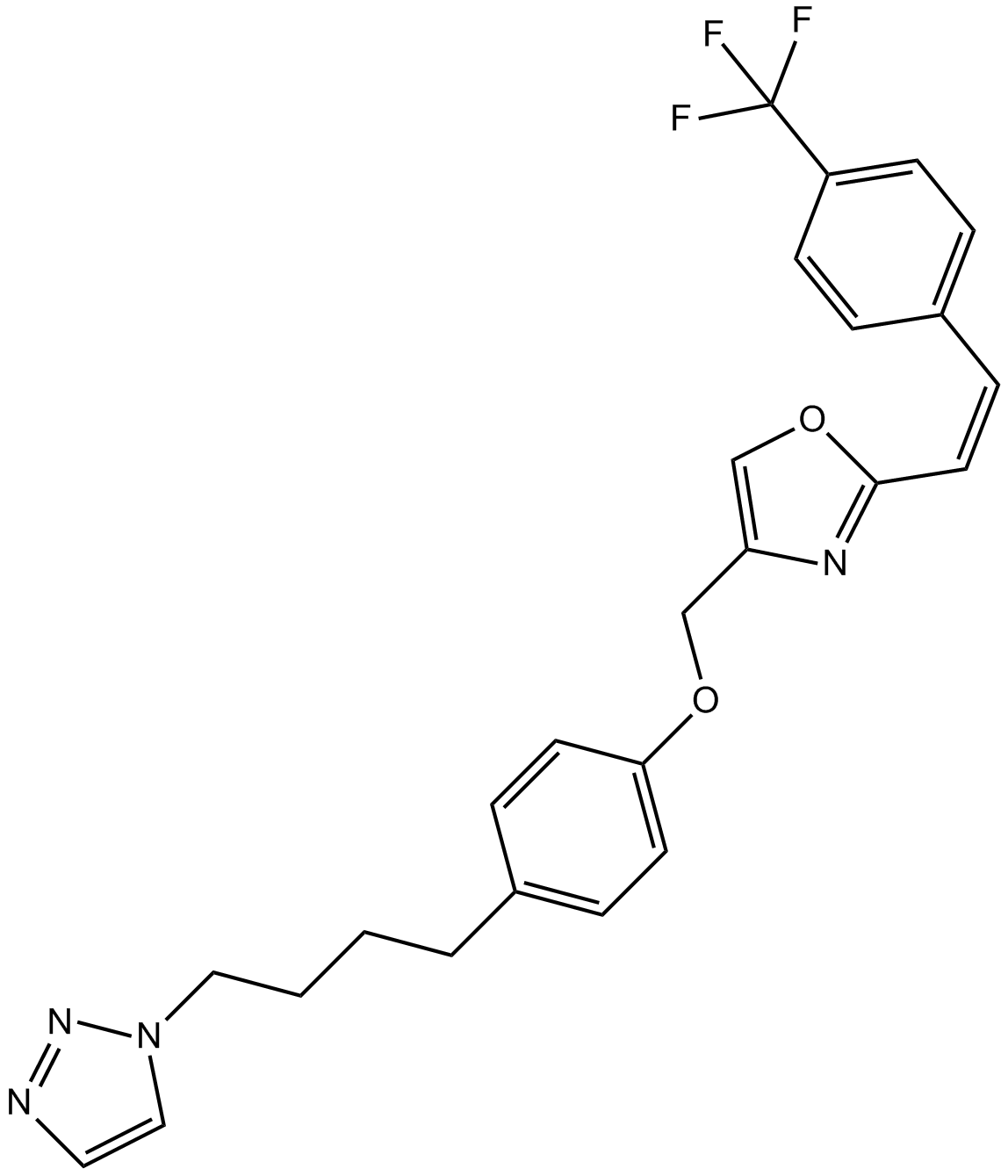
Mubritinib (TAK 165)
Mubritinib (TAK-165, CAS No. 366017-09-6) has its core target on mitochondrial electron transport chain complex I (ETC Complex I, NADH dehydrogenase). It inhibits the function of the complex by binding to its active site in a ubiquinone-dependent manner. Meanwhile, it can suppress the binding of the latency-associated nuclear antigen (LANA) protein of Kaposi’s sarcoma-associated herpesvirus (KSHV) to viral terminal repeat (TR) sequences. The originally reported target HER2 has no actual functional relevance in diseases such as acute myeloid leukemia (AML) and primary effusion lymphoma (PEL). Its key concentration data are clearly defined: the in vitro IC₅₀ for complex I inhibition is 51 nM (cell-free assay); the median GI₅₀ in AML cells is 374 nM (147–153 nM for the MLL-AF9 subtype); the GI₅₀ in KSHV-positive PEL cells ranges from 7.5 to 17.1 nM (7.5 nM in BC1 cells, 13.45 nM in BC3 cells, 17.1 nM in BCBL1 cells); the original IC₅₀ for HER2 inhibition is 0.35 μM, which has no clinical relevance.
Commonly used application concentrations are as follows: in in vitro cell assays, 0.1–10 μM for AML cells, 7.5–15 nM for PEL cells, and nanomolar range (10–100 nM) for complex I inhibition assays; in animal experiments, 20–25 mg/kg/day via intraperitoneal injection or oral administration. The therapeutically effective concentration corresponds to preclinical dosing regimens: oral administration of 20 mg/kg (AML model) or 25 mg/kg (PEL model) in mice can maintain the serum concentration at an effective level for 48 consecutive hours. Mubritinib was previously used in Phase I clinical trials for solid tumors, and is now proposed to be repurposed for chemotherapy-resistant AML and PEL, with a recommended reference dose of 20–25 mg/kg once daily. Its biological activities are manifested as inhibiting oxidative phosphorylation (OXPHOS), inducing oxidative stress and apoptosis, and selectively killing chemotherapy-resistant AML cells (subtypes with high HOX gene expression or NPM1/FLT3/DNMT3A mutations) as well as PEL cells, without affecting normal CD34⁺ hematopoietic stem cells. It exhibits good tolerability in animal experiments and can significantly prolong the survival time of tumor-bearing mice.
References:
[1] Baccelli I, Gareau Y, Lehnertz B, Gingras S, Spinella JF, Corneau S, Mayotte N, Girard S, Frechette M, Blouin-Chagnon V, Leveillé K, Boivin I, MacRae T, Krosl J, Thiollier C, Lavallée VP, Kanshin E, Bertomeu T, Coulombe-Huntington J, St-Denis C, Bordeleau ME, Boucher G, Roux PP, Lemieux S, Tyers M, Thibault P, Hébert J, Marinier A, Sauvageau G. Mubritinib Targets the Electron Transport Chain Complex I and Reveals the Landscape of OXPHOS Dependency in Acute Myeloid Leukemia. Cancer Cell. 2019 Jul 8;36(1):84-99.e8. doi: 10.1016/j.ccell.2019.06.003. PMID: 31287994.
[2] Stephenson ZA, Harvey RF, Pryde KR, Mistry S, Hardy RE, Serreli R, Chung I, Allen TE, Stoneley M, MacFarlane M, Fischer PM, Hirst J, Kellam B, Willis AE. Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I. Elife. 2020 May 20;9:e55845. doi: 10.7554/eLife.55845. PMID: 32432547; PMCID: PMC7316505.
[3] Calderon A, Soldan SS, De Leo A, Deng Z, Frase DM, Anderson EM, Zhang Y, Vladimirova O, Lu F, Leung JC, Murphy ME, Lieberman PM. Identification of Mubritinib (TAK 165) as an inhibitor of KSHV driven primary effusion lymphoma via disruption of mitochondrial OXPHOS metabolism. Oncotarget. 2020 Nov 17;11(46):4224-4242. doi: 10.18632/oncotarget.27815. PMID: 33245718; PMCID: PMC7679036.
[4] Dong J, Zhu D, Chen M, Wang T, Gao Y, Liu W. Mubritinib enhanced the inhibiting function of cisplatin in lung cancer by interfering with mitochondrial function. Thorac Cancer. 2022 May;13(10):1513-1524. doi: 10.1111/1759-7714.14425. Epub 2022 Apr 16. PMID: 35429141; PMCID: PMC9108040.
[5] Li XY, Qian XH, Zhu J, Li YH, Lin QQ, Li S, Xue WH, Jian LY, Meng FH. Synthesis and evaluation of novel HER-2 inhibitors to exert anti-breast cancer ability through epithelial-mesenchymal transition (EMT) pathway. Eur J Med Chem. 2022 Jul 5;237:114325. doi: 10.1016/j.ejmech.2022.114325. Epub 2022 Mar 29. PMID: 35452936.
[6] Menezes TM, Seabra G, Neves JL. Molecular Recognition Study toward the Mitochondrial Electron Transport Chain Inhibitor Mubritinib and Human Serum Albumin. Mol Pharm. 2023 Aug 7;20(8):4021-4030. doi: 10.1021/acs.molpharmaceut.3c00187. Epub 2023 Jun 29. PMID: 37382244.
[7] Chiem K, Nogales A, Lorenzo M, Morales Vasquez D, Xiang Y, Gupta YK, Blasco R, de la Torre JC, Martínez-Sobrido L. Identification of In Vitro Inhibitors of Monkeypox Replication. Microbiol Spectr. 2023 Aug 17;11(4):e0474522. doi: 10.1128/spectrum.04745-22. Epub 2023 Jun 6. Erratum in: Microbiol Spectr. 2024 Jan 11;12(1):e0362123. doi: 10.1128/spectrum.03621-23. PMID: 37278625; PMCID: PMC10434227.
| Storage | Store at -20°C |
| M.Wt | 468.47 |
| Cas No. | 366017-09-6 |
| Formula | C25H23F3N4O2 |
| Solubility | insoluble in H2O; ≥76.9 mg/mL in DMSO with gentle warming; ≥3.09 mg/mL in EtOH with gentle warming and ultrasonic |
| Chemical Name | 4-[[4-[4-(triazol-1-yl)butyl]phenoxy]methyl]-2-[(E)-2-[4-(trifluoromethyl)phenyl]ethenyl]-1,3-oxazole |
| SDF | Download SDF |
| Canonical SMILES | C(=C/C1=CC=C(C(F)(F)F)C=C1)\C2=NC(COC3=CC=C(CCCCN4C=CN=N4)C=C3)=CO2 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure