MT-DADMe-lmmA
MT-DADMe-ImmA (CAS 653592-04-2) targets human 5'-methylthioadenosine phosphorylase (MTAP)—a key enzyme in 5'-methylthioadenosine (MTA) metabolism/S-adenosylmethionine (SAM) salvage and a validated anticancer target. As a second-generation transition state (TS) analogue inhibitor, it exhibits high binding affinity with an equilibrium dissociation constant (Ki∗) of 90 pM (11-fold stronger than MT-ImmA), and 5'-substituted derivatives (e.g., pClPhT-DADMe-ImmA) achieve a Ki∗ of 10 pM. In immunodeficient mice, it induces head and neck cancer regression and inhibits lung cancer growth/metastasis with low toxicity, while forming a highly thermally stable ternary complex (MTAP/phosphate/inhibitor, Tm~95 °C, +28 °C vs. apo MTAP).
It acts via a two-step slow-onset tight-binding mechanism: reversible EI complex formation (Ki=1.7 nM) followed by conformational rearrangement to a stable E*I complex. Its core mechanism mimics MTAP’s dissociative SN1 transition state: a cationic nitrogen (simulating cationic ribosyl moiety) and protonated 9-deazaadenine (adenine leaving group) are separated by a 2.8 Å methylene bridge (matching late TS geometry). Binding to apo MTAP is enthalpy-driven, switching to entropy-driven upon ternary complex formation—triggered by phosphate-inhibitor cationic nitrogen ion pairing (-3.6 kcal/mol binding free energy) and 9-deazaadenine-Asp220 hydrogen bonding (-1.7 kcal/mol). Complete inhibition requires occupancy of all three MTAP homotrimer active sites, with synergistic binding with phosphate enhancing target specificity.
References:
[1] Guan R, Tyler PC, Evans GB, Schramm VL. Thermodynamic analysis of transition-state features in picomolar inhibitors of human 5'-methylthioadenosine phosphorylase. Biochemistry. 2013 Nov 19;52(46):8313-22. doi: 10.1021/bi401188w. Epub 2013 Nov 8. PMID: 24148083; PMCID: PMC3870587.
| Storage | Store at -20°C |
| M.Wt | 293.40 |
| Cas No. | 653592-04-2 |
| Formula | C13H19N5OS |
| Synonyms | MTDIA; Methylthio-DADMe-Immucillin A |
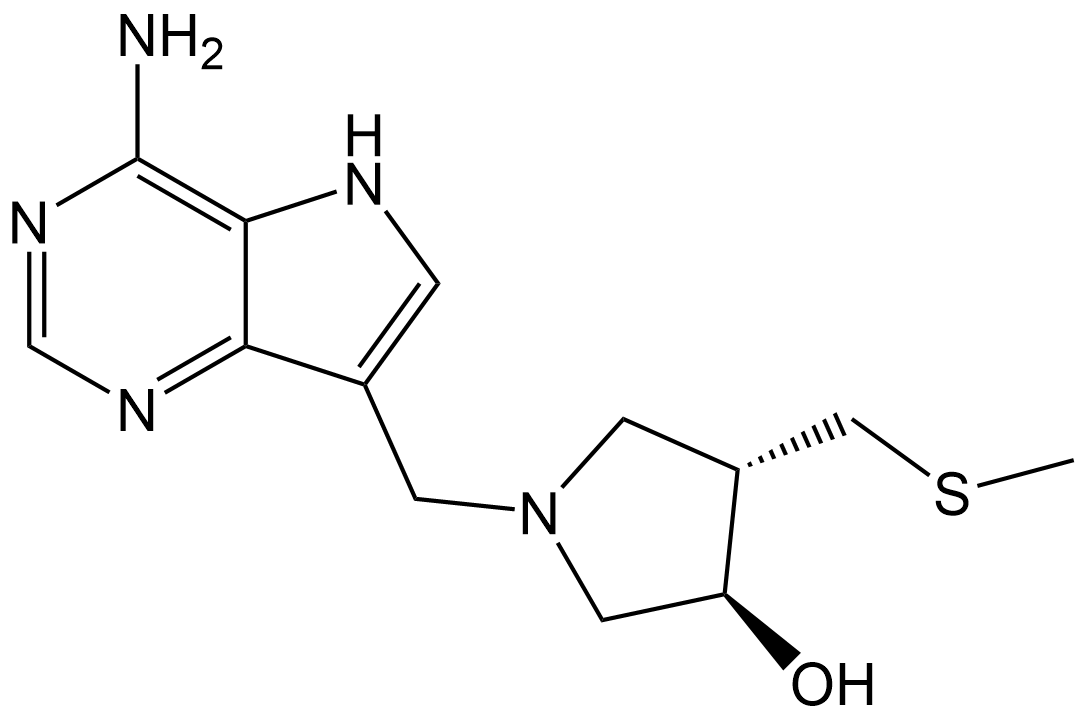
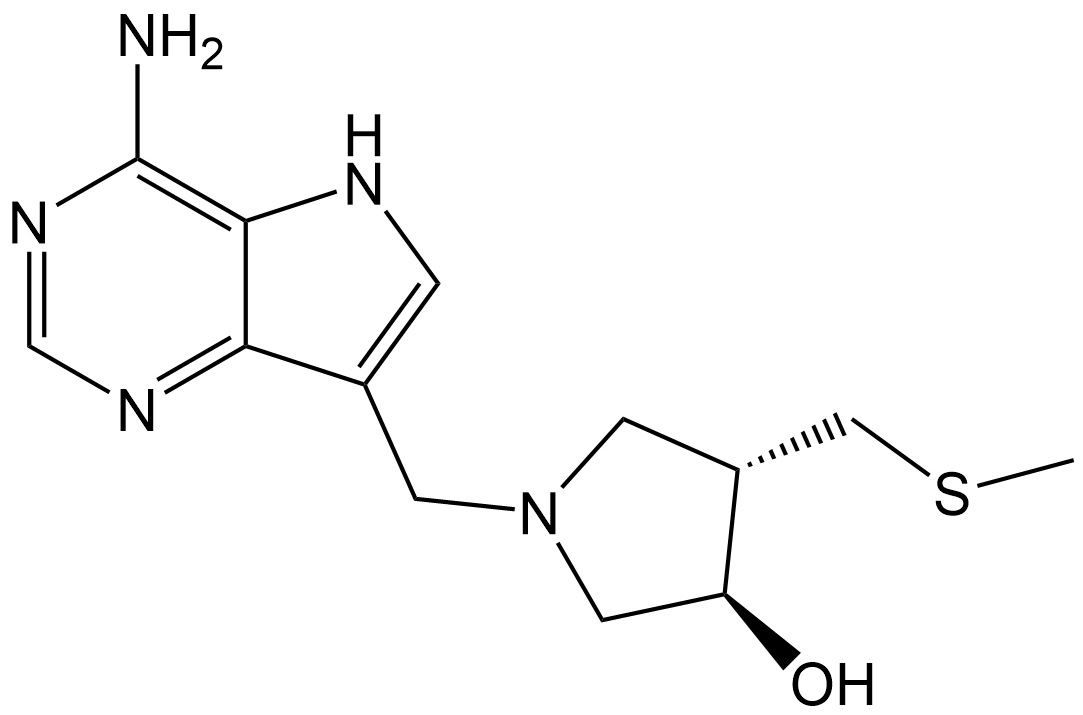
| Chemical Name | (3R,4S)-1-((4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)methyl)-4-((methylthio)methyl)pyrrolidin-3-ol |
| SDF | Download SDF |
| Canonical SMILES | NC1=C2C(C(CN3C[C@H](O)[C@@H](CSC)C3)=CN2)=NC=N1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure