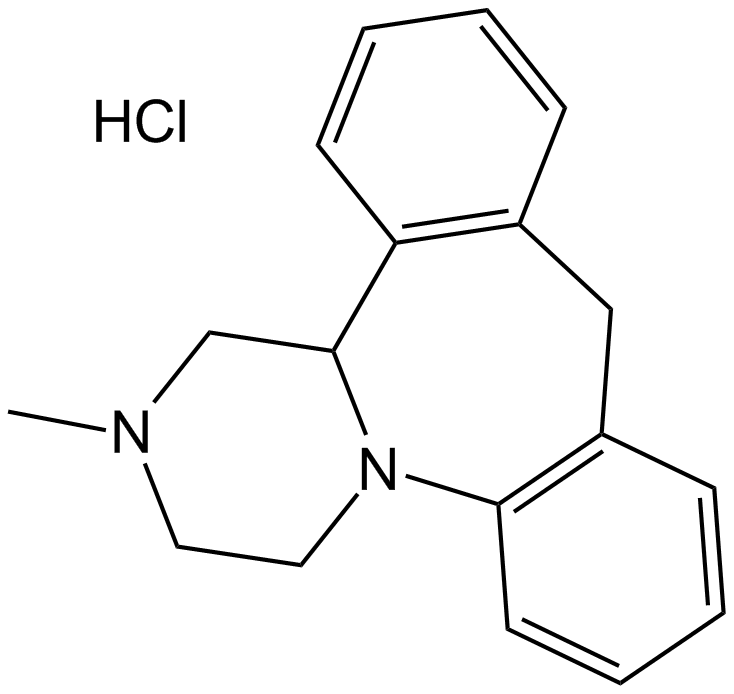
Mianserin HCl
Mianserin Hydrochloride (CAS No. 21535-47-7) is a tetracyclic antidepressant. Its core targets are noradrenergic receptors and specific serotonin (5-HT) receptors. It lacks monoamine oxidase inhibitory activity and does not interfere with amine reuptake. Additionally, it acts on the pathogenic Leishmania donovani by depleting ergosterol, and forms inclusion complexes with β-cyclodextrin (β-CD) and its methylated derivative (DM-β-CD) at a stoichiometry of 1:1 or 1:1.5. The binding constant (K) with DM-β-CD is 1690 M⁻¹ with a Gibbs free energy (ΔG) of -18.42 kJ·mol⁻¹, while the binding constant with β-CD is 1320 M⁻¹.
Common application concentrations are as follows: 200 μM for mianserin hydrochloride (MIA) and 0.1–1000 μM for DM-β-CD in cytotoxicity assays; 0.79 mM for MIA and 15 mM for DM-β-CD in isothermal titration calorimetry (ITC) experiments; 0.6 mM for MIA and 0.1–10 mM for DM-β-CD in circular dichroism spectroscopy assays.
The clinically effective therapeutic concentration corresponds to an oral dosage of 10–20 mg per administration, three times daily (total daily dose: 30–60 mg). After 14 days of treatment, the plasma concentration ranges from 18.3 to 72.7 ng/mL, with an average of 50.7 μg/L.
Its biological activities are manifested as a significant improvement in depressive symptoms (evidenced by reduced scores on the Beck Depression Inventory and Hamilton Depression Rating Scale), increased sleep duration and quality, inhibitory effects on Leishmania donovani, and blood glucose stabilization. When complexed with DM-β-CD, it exhibits enhanced cytotoxicity against hamster B14 cells (resulting in a cell viability rate of only 6–7%). It has favorable clinical tolerability, with a lower incidence of adverse effects than amitriptyline. It is indicated for the treatment of depression and holds potential applications in antipathogenic therapy and blood glucose regulation.
References:
[1] Coppen A, Gupta R, Montgomery S, Ghose K, Bailey J, Burns B, de Ridder JJ. Mianserin hydrochloride: a novel antidepressant. Br J Psychiatry. 1976 Oct;129:342-5. doi: 10.1192/bjp.129.4.342. PMID: 974442.
[2] Smith AH, Naylor GS, Moody JP. Placebo-controlled double-blind trial of mianserin hydrochloride. Br J Clin Pharmacol. 1978;5 Suppl 1(Suppl 1):67S-70S. PMID: 341946; PMCID: PMC1429200.
[3] Belica-Pacha S, Małecka M, Daśko M, Miłowska K, Bryszewska M, Budryn G, Oracz J, Pałecz B. The Interaction of Heptakis (2,6-di-O-Methyl)-β-cyclodextrin with Mianserin Hydrochloride and Its Influence on the Drug Toxicity. Int J Mol Sci. 2021 Aug 30;22(17):9419. doi: 10.3390/ijms22179419. PMID: 34502332; PMCID: PMC8430726.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 300.83 |
| Cas No. | 21535-47-7 |
| Formula | C18H20N2·HCl |
| Solubility | ≥15.04 mg/mL in DMSO; ≥2.71 mg/mL in H2O with gentle warming and ultrasonic; ≥8.23 mg/mL in EtOH with ultrasonic |
| Chemical Name | 2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyrazino[1,2-a]azepine hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | CN(CC1)CC(c2c(C3)cccc2)N1c1c3cccc1.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure