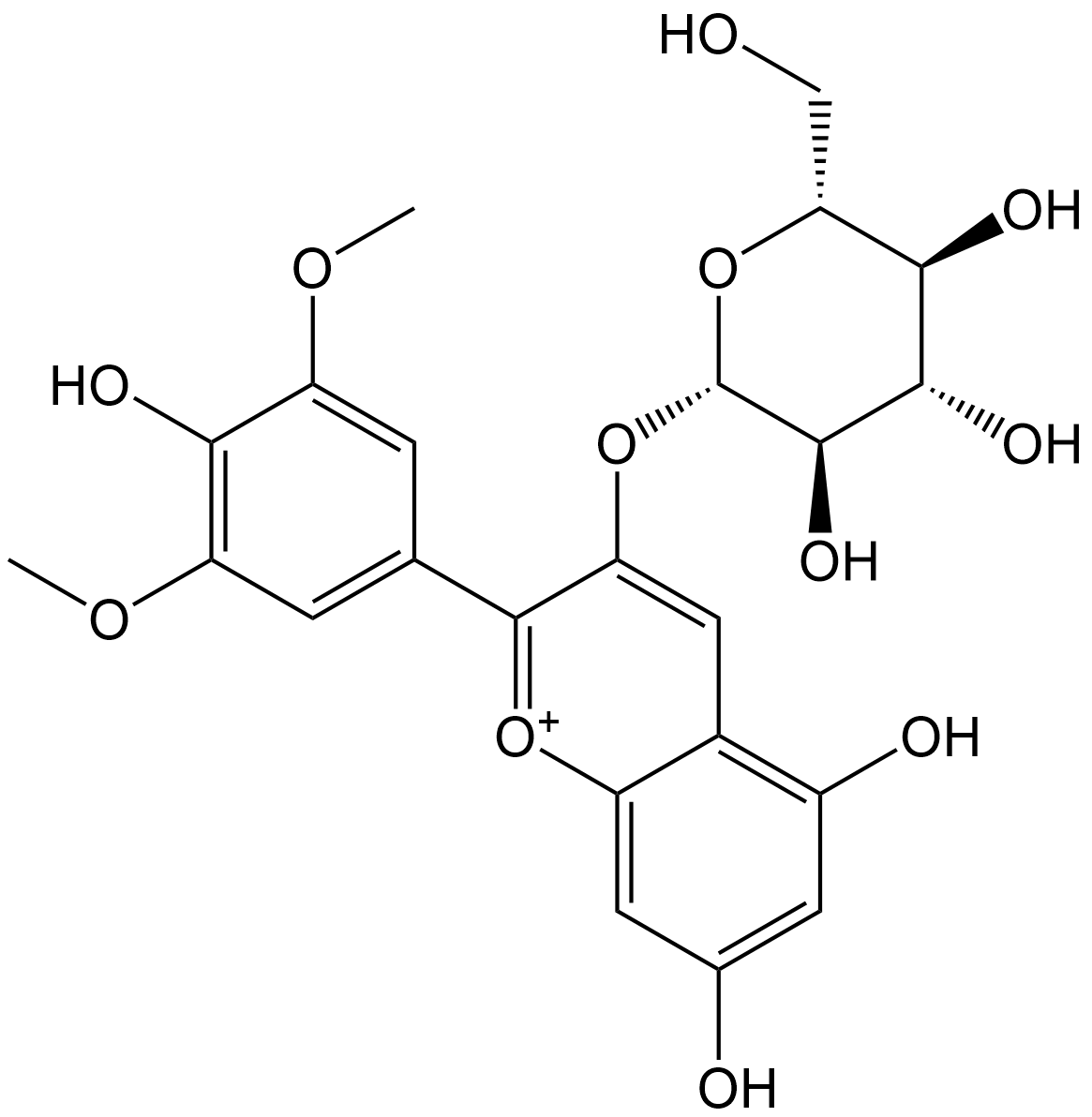
Malvidin 3-O-beta-D-glucopyranoside
Malvidin 3-O-glucoside (CAS 18470-06-9) is a major anthocyanin component in red grapes (Vitis vinifera L.) and red wines, with its core target being the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling pathway. It also undergoes intermolecular copigmentation with phenolic copigments (e.g., gallic acid, (−)-epicatechin, quercetin-3-O-glucoside) and exhibits multiple bioactivities, including neuroprotection, antioxidation, anti-apoptosis, anti-inflammation, and food color stabilization.
In terms of neuroprotection, at a concentration of 50 μM, it significantly alleviates the toxicity of human microglial HMC3 cells co-induced by α-synuclein (α-syn, 8 μg/mL) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, 2 mM). It activates the Nrf2/HO-1 pathway to upregulate the transcriptional and translational levels of antioxidant enzymes (catalase [CAT], superoxide dismutase [SOD], glutathione peroxidase [GPx]) and reduce reactive oxygen species (ROS) accumulation. Meanwhile, it downregulates the expression of pro-apoptotic genes (Bax, casp-3, casp-8) and upregulates the anti-apoptotic gene Bcl-2 to inhibit cell apoptosis, and also downregulates pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) while upregulating anti-inflammatory cytokines (IL-4, TGF-β).
For color stabilization, it forms 1:1 binding copigmentation complexes with phenolic copigments, with a maximum binding constant (K) of 49259.16 L/mol (when bound to quercetin-3-O-glucoside). It enhances the stability and saturation of solution color via hydrogen bonds and van der Waals forces, and its copigmentation efficacy is significantly superior to that of acetylated derivatives due to the absence of steric hindrance from acetylation. Additionally, it possesses potential hypoglycemic and hypolipidemic activities and exhibits excellent biocompatibility, making it a potential agent for preventing/treating neurodegenerative diseases (e.g., Parkinson’s disease), a natural food color stabilizer, and a functional ingredient for regulating metabolic disorders.
References:
[1] Zhao X, Zhang X, He X, Duan C, He F. Acetylation of Malvidin-3-O-glucoside Impedes Intermolecular Copigmentation: Experimental and Theoretical Investigations. J Agric Food Chem. 2021 Jul 14;69(27):7733-7741. doi: 10.1021/acs.jafc.1c02378. Epub 2021 Jun 30. PMID: 34192464.
[2] Sood R, Sanjay, Kang SU, Yoon NY, Lee HJ. Malvidin-3-O-Glucoside Mitigates α-Syn and MPTP Co-Induced Oxidative Stress and Apoptosis in Human Microglial HMC3 Cells. Int J Mol Sci. 2024 Nov 27;25(23):12733. doi: 10.3390/ijms252312733. PMID: 39684444; PMCID: PMC11641650.
| Storage | -20℃, sealed storage, away from moisture and light |
| M.Wt | 493.44 |
| Cas No. | 18470-06-9 |
| Formula | C23H25O12+ |
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-3-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)chromenylium |
| SDF | Download SDF |
| Canonical SMILES | OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](OC2=CC3=C(O)C=C(O)C=C3[O+]=C2C4=CC(OC)=C(O)C(OC)=C4)O1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure