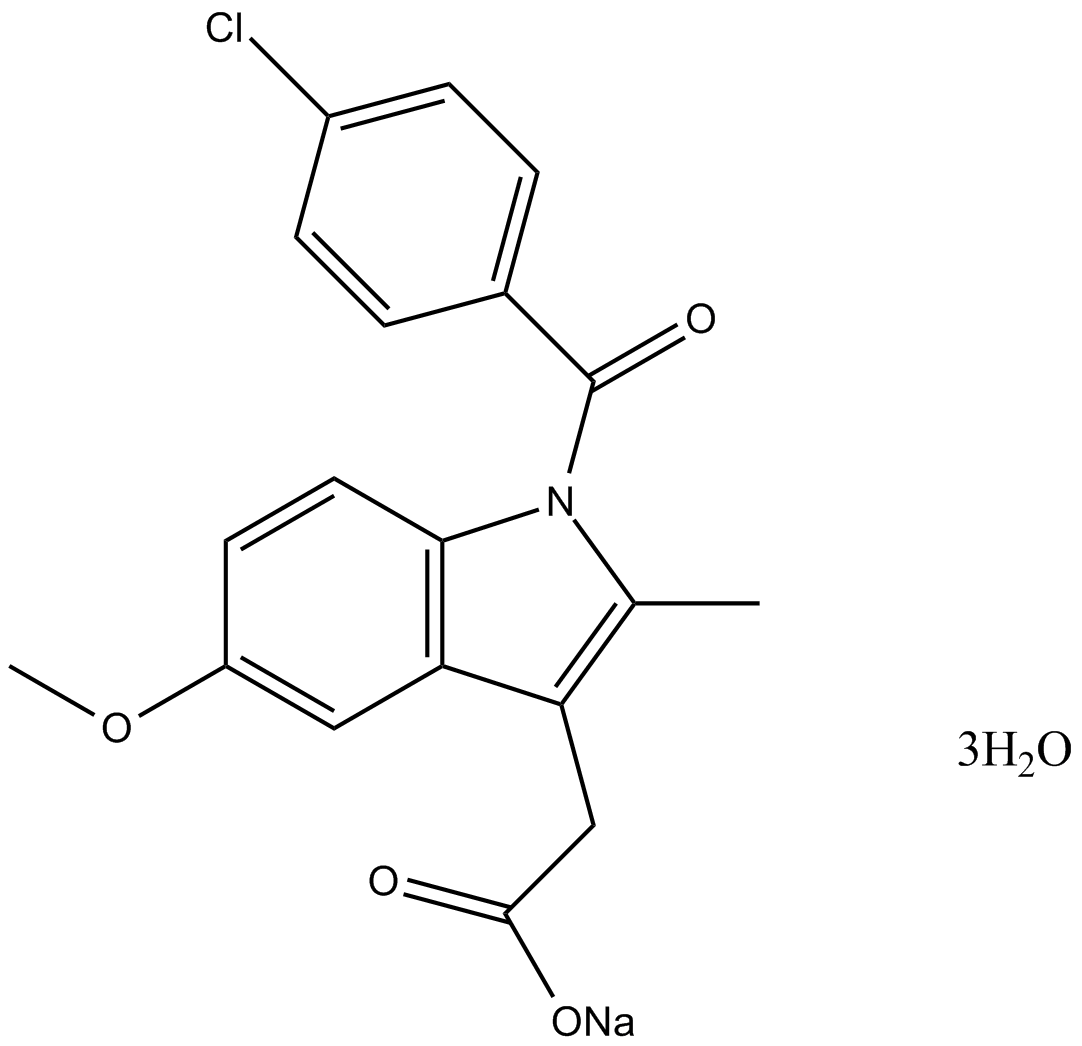
Indomethacin sodium trihydrate
Indomethacin Sodium Trihydrate (CAS No. 74252-25-8) is the trihydrated sodium salt form of Indometacin (CAS No. 53-86-1, Catalog No.: A8449). As a non-steroidal anti-inflammatory drug (NSAID), its core targets include cyclooxygenase 1/2 (COX-1/COX-2, non-selective inhibition), the Wnt/β-catenin signaling pathway, and glycogen synthase kinase 3β (GSK3β). Meanwhile, it can affect targets related to follicular rupture by inhibiting prostaglandin synthesis and regulate pathways associated with oligodendrocyte differentiation; its activity depends on the physiological effects mediated by target inhibition. Commonly used application concentrations: 2.5~200 μM in cell experiments (e.g., 2.5 μM in oligodendrocyte differentiation experiments, 10~200 mg/L in pancreatic stellate cell proliferation experiments), and 2.5 mg/kg/day via intraperitoneal injection in animal experiments (cuprizone-induced demyelination model). The clinically effective therapeutic concentration corresponds to oral dosages: a single dose of 50 mg for acute pain; a maximum daily dose of 200 mg (administered in multiple divided doses) for chronic rheumatic diseases, gout, etc.; and 50 mg three times daily in modified natural cycle IVF (from the day of HCG trigger to before oocyte retrieval). Its biological activities include anti-inflammation, analgesia, antipyrexia, inhibiting the proliferation and migration of pancreatic stellate cells, and promoting the differentiation of oligodendrocytes and myelin regeneration in humans and mice. In the IVF subgroup without an LH surge, it can reduce premature ovulation. Regarding safety, common adverse reactions include gastrointestinal discomfort and headaches; long-term use requires attention to the risks of renal injury and gastrointestinal ulcers. It is indicated for the treatment of rheumatic diseases, gout, certain types of pain, and specific IVF scenarios.
References:
[1] Mason L, Edwards J, Moore RA, McQuay HJ. Single dose oral indometacin for the treatment of acute postoperative pain. Cochrane Database Syst Rev. 2004 Oct 18;2004(4):CD004308. doi: 10.1002/14651858.CD004308.pub2. PMID: 15495100; PMCID: PMC4171122.
[2] Rijken-Zijlstra TM, Haadsma ML, Hammer C, Burgerhof JG, Pelinck MJ, Simons AH, van Echten-Arends J, Arts JG, Land JA, Groen H, Hoek A. Effectiveness of indometacin to prevent ovulation in modified natural-cycle IVF: a randomized controlled trial. Reprod Biomed Online. 2013 Sep;27(3):297-304. doi: 10.1016/j.rbmo.2013.05.009. Epub 2013 May 22. PMID: 23876971.
[3] Preisner A, Albrecht S, Cui QL, Hucke S, Ghelman J, Hartmann C, Taketo MM, Antel J, Klotz L, Kuhlmann T. Non-steroidal anti-inflammatory drug indometacin enhances endogenous remyelination. Acta Neuropathol. 2015 Aug;130(2):247-61. doi: 10.1007/s00401-015-1426-z. Epub 2015 May 6. PMID: 25943886.
[4] Sun L, Chen K, Jiang Z, Chen X, Ma J, Ma Q, Duan W. Indometacin inhibits the proliferation and activation of human pancreatic stellate cells through the downregulation of COX-2. Oncol Rep. 2018 May;39(5):2243-2251. doi: 10.3892/or.2018.6321. Epub 2018 Mar 16. PMID: 29565462.
| Storage | Store at -20°C |
| M.Wt | 433.82 |
| Cas No. | 74252-25-8 |
| Formula | C19H21ClNNaO7 |
| Solubility | ≥87mg/mL in DMSO |
| Chemical Name | sodium 2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetate |
| SDF | Download SDF |
| Canonical SMILES | Cc1c(CC([O-])=O)c(cc(cc2)OC)c2[n]1C(c(cc1)ccc1Cl)=O.[Na+] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure