IL-18, human recombinant protein
Interleukin-18 (IL-18) is a proinflammatory cytokine in the IL-1 family that exerts distinct immune effects depending on the local cytokine environment. It is expressed as a 24 kDa precursor by endothelial and epithelial cells, keratinocytes, gamma δ T cells, and phagocytes. The precursor is activated intracellularly by Caspase-1 mediated proteolysis to release the 17 kDa mature cytokine. The precursor can also be released by necrotic cells for extracellular cleavage by multiple proteases. IL-18 activation is induced by infection or tissue damage and contributes to disease pathology in chronic inflammation. IL-18 binds to the widely expressedIL-18 R alpha which recruits IL-18 R beta to form the signaling receptor complex. Its bioactivity is negatively regulated by interactions with IL-18 binding proteins and virally encoded IL-18BP homologs. In the presence of IL-12 or IL-15, IL-18 enhances anti-viral Th1 immune responses by inducing IFN-gamma production and the cytolytic activity of CD8+ T cells and NK cells. In the absence of IL-12 or IL-15, however, IL-18 promotes production of the Th2 cytokines IL-4 and IL-13 by CD4+ T cells and basophils. In the presence of IL-1 beta or IL-23, IL-18 induces the antigen-independent production of IL-17 by gamma δ T cells and CD4+ T cells. IL-18 also promotes myeloid dendritic cell maturation and triggers neutrophil respiratory burst. In cancer, IL-18 exhibits diverse activities including enhancing anti-tumor immunity, inhibiting or promoting angiogenesis, and promoting tumor cell metastasis. Mature human IL-18 shares approximately 63% amino acid sequence identity with mouse and rat IL-18. Alternative splicing in human ovarian cancer generates an isoform that is resistant to Caspase-1 activation. A cell surface form can be expressed on M-CSF induced macrophages and released in response to bacterial endotoxin.
|
Gene ID |
3606 |
|
Accession # |
Q14116 |
|
Alternate Names |
|
|
Source |
Escherichia coli. |
|
M.Wt |
Approximately 18.2 kDa, a single non-glycosylated polypeptide chain containing 157 amino acids. |
|
AA Sequence |
YFGKLESKLS VIRNLNDQVL FIDQGNRPLF EDMTDSDCRD NAPRTIFIIS MYKDSQPRGM AVTISVKCEK ISTLSCENKI ISFKEMNPPD NIKDTKSDII FFQRSVPGHD NKMQFESSSY EGYFLACEKE RDLFKLILKK EDELGDRSIM FTVQNED |
|
Appearance |
Sterile Filtered White lyophilized (freeze-dried) powder. |
|
Stability & Storage |
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. - 12 months from date of receipt, -20 to -70 °C as supplied. - 1 month, 2 to 8 °C under sterile conditions after reconstitution. - 3 months, -20 to -70 °C under sterile conditions after reconstitution. |
|
Formulation |
Lyophilized from a 0.2 μm filtered solution in PBS, pH 7.0. |
|
Reconstitution |
We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile distilled water or aqueous buffer containing 0.1 % BSA to a concentration of 0.1-1.0 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 ℃. Further dilutions should be made in appropriate buffered solutions. |
|
Biological Activity |
Test in processing. |
|
Shipping Condition |
Gel pack. |
|
Handling |
Centrifuge the vial prior to opening. |
|
Usage |
For Research Use Only! Not to be used in humans. |
Quality Control & DataSheet
- View current batch:
-
Purity > 95 % by SDS-PAGE analyses.
- Datasheet
Endotoxin: Less than 0.1 EU/μg of rHuIL-18 as determined by LAL method.
Related Biological Data
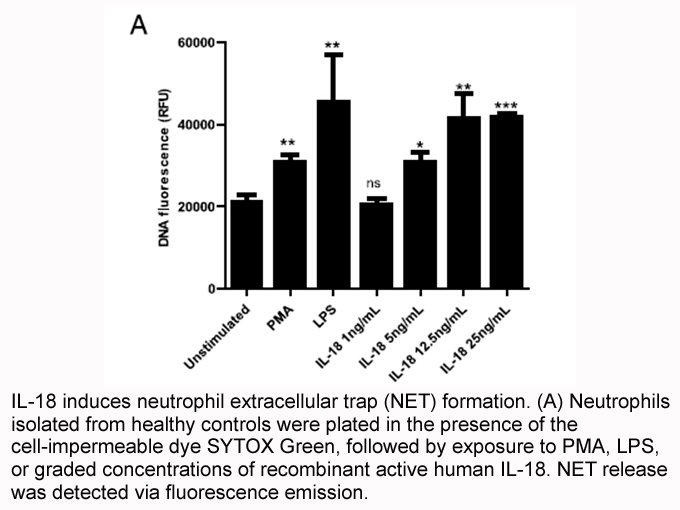
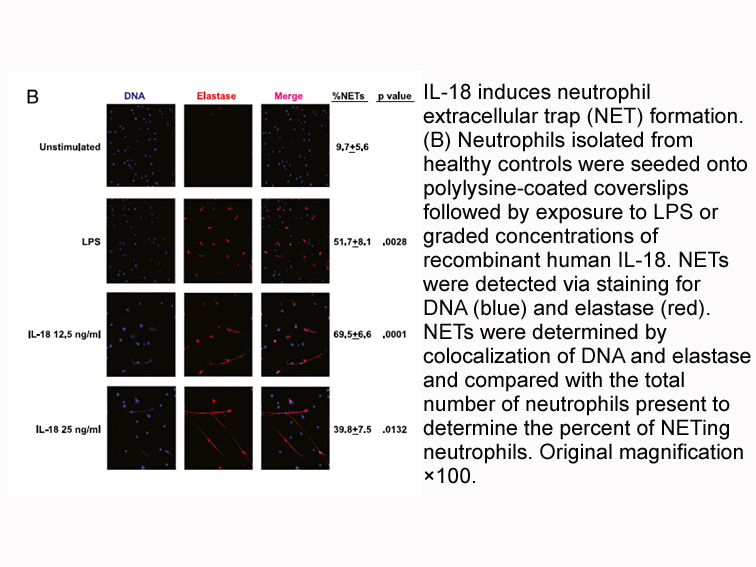
Related Biological Data
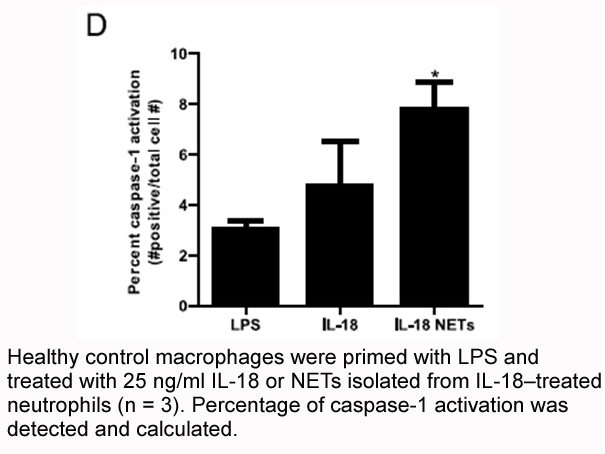
Related Biological Data