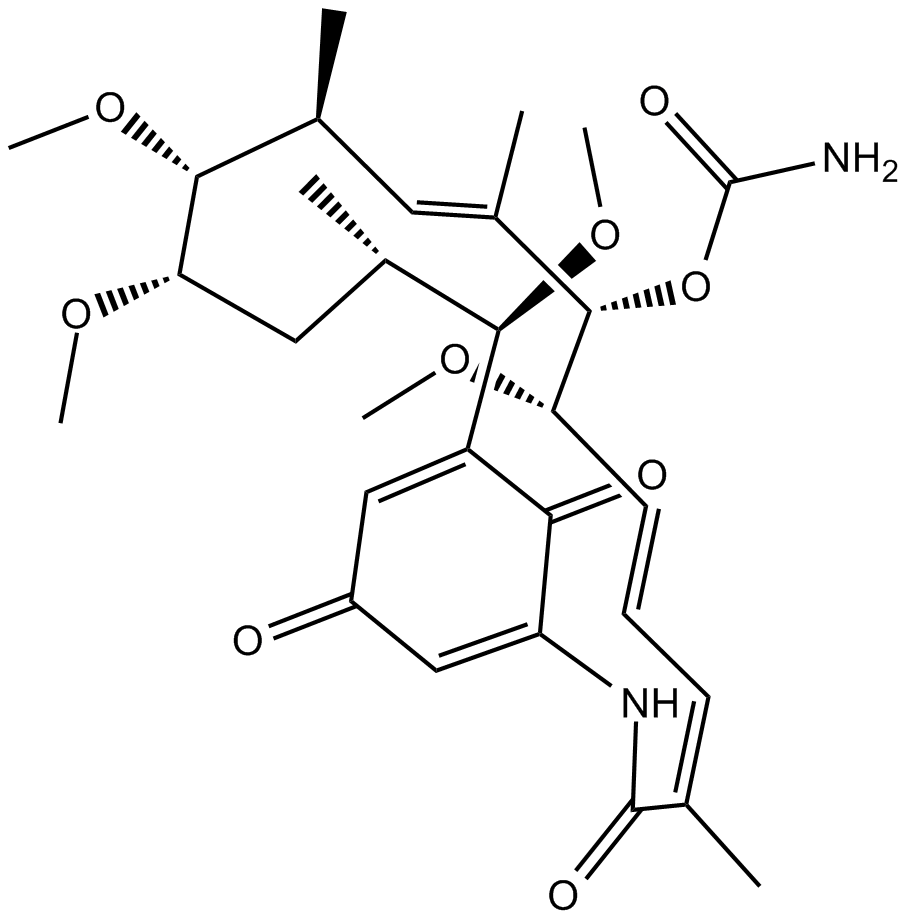
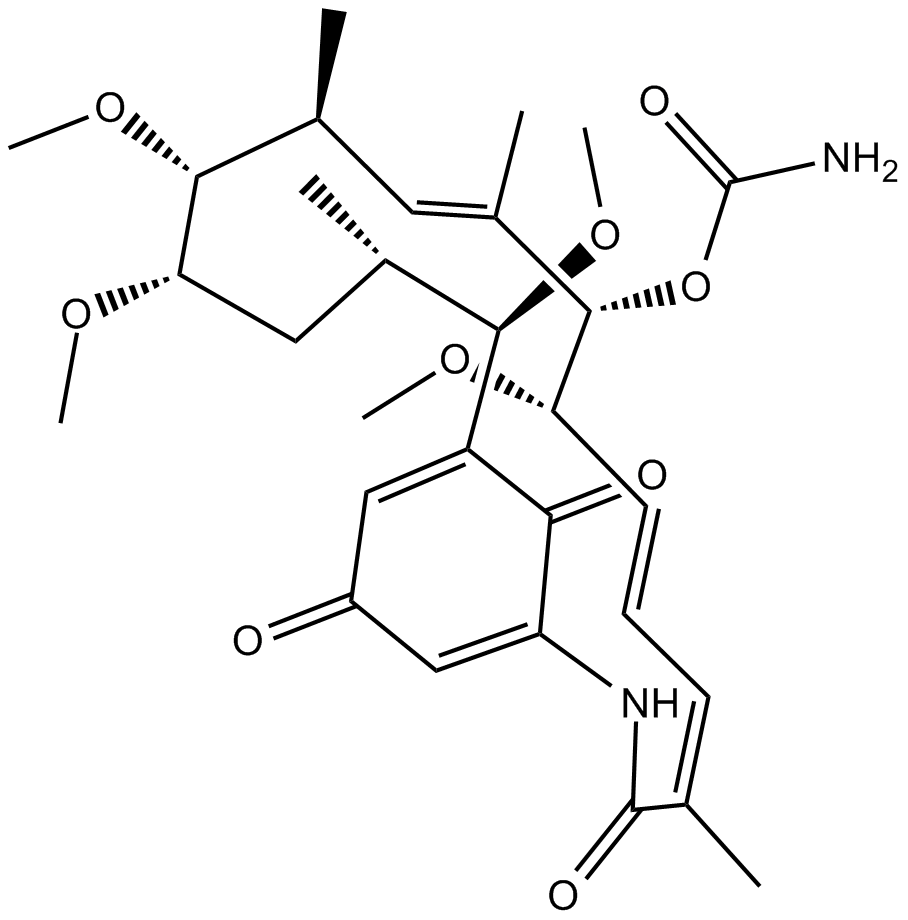
Herbimycin A
Herbimycin A is an antibiotic and a selective inhibitor of non-receptor tyrosine kinases [1].
Antibiotics are a type of antimicrobial used in the treatment of bacterial infection. Non-receptor tyrosine kinases (nRTKs) are cytoplasmic enzymes and catalyse the transfer of a phosphate group from a nucleoside triphosphate donor to tyrosine residues in proteins.
Herbimycin A is a selective non-receptor tyrosine kinases inhibitor. Herbimycin A bound to the reactive SH domains of p60v-src and p210BCR-ABL and inactivated their activity [1].
In a retinopathy prematurity rat model, Herbimycin A inhibited capillary tube formation and bovine retinal microvascular endothelial cell proliferation induced by vascular endothelial growth factor in a dose-dependent way. Also, Herbimycin A reduced pre-retinal neovascularization by 63% and 41% in oxygen-treated rats and herbimycin-injected eyes, respectively [2]. In rats exposed to heat stress, Herbimycin A exhibited thermotolerance and significantly reduced apoptosis of hepatocytes. Also, Herbimycin A inhibited caspase-3 activation [3].
References:
[1]. Fukazawa H, Uehara Y, Murakami Y, et al. Labeling of v-Src and BCR-ABL tyrosine kinases with [14C]herbimycin A and its use in the elucidation of the kinase inactivation mechanism. FEBS Lett, 1994, 340(3): 155-158.
[2]. McCollum GW, Rajaratnam VS, Bullard LE, et al. Herbimycin A inhibits angiogenic activity in endothelial cells and reduces neovascularization in a rat model of retinopathy of prematurity. Exp Eye Res, 2004, 78(5): 987-995.
[3]. Sachidhanandam SB, Lu J, Low KS, et al. Herbimycin A attenuates apoptosis during heat stress in rats. Eur J Pharmacol, 2003, 474(1): 121-128.
| Physical Appearance | A yellow lyophilisate |
| Storage | Store at -20°C |
| M.Wt | 574.67 |
| Cas No. | 70563-58-5 |
| Formula | C30H42N2O9 |
| Solubility | Soluble in DMSO |
| Chemical Name | (4Z,6E,8S,9S,10E,12S,13R,14S,16S,17R)-8,13,14,17-tetramethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate |
| SDF | Download SDF |
| Canonical SMILES | O=C(C(NC1=O)=C2)C([C@@H]([C@@H](C)C[C@@H]([C@@H]([C@@H](C)/C=C(C)/[C@@H]([C@H](/C=C/C=C1/C)OC)OC(N)=O)OC)OC)OC)=CC2=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure