GYKI 47261 dihydrochloride
GYKI 47261 dihydrochloride is a selective and non-competitive antagonist of AMPA receptor with IC50 value of 2.5 μM [1].
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor) is an ionotropic transmembrane receptor for glutamate and mediates fast synaptic transmission in the central nervous system. AMPA receptors are oligomeric assemblies of four protein subunits, GluR1-4.
GYKI 47261 dihydrochloride is a selective and non-competitive AMPA receptor antagonist. In isolated cerebellar Purkinje cells, GYKI 47261 (10 μM) inhibited currents induced by kainate or AMPA in a non-competitive way [1]. In rat hepatocytes, GYKI-47261 (10 μM) significantly increased CYP2E1 activity. In human hepatocytes, GYKI-47261 produced the maximal induction at 0.01 μM [2].
In mice, GYKI 47261 induced muscle relaxant effects with ED50 values of 15.8-36.5 mg/kg. In a transient focal ischemia rat model, GYKI 47261 significantly reduced infarct size by 62.3-67.4%. In mice, GYKI 47261 effectively reduced oxotremorine-induced tremor and inhibited MPTP-induced neurotoxicity [1]. In Parkinson’s disease rat model, administration of GYKI-47261 and MK-801 completely normalized the response shortening induced by levodopa. In monkeys, administration of amantadine and GYKI-47261 significantly reduced dyskinesias induced by levodopa by 51% [3].
References:
[1]. Abrahám G, Sólyom S, Csuzdi E, et al. New non competitive AMPA antagonists. Bioorg Med Chem, 2000, 8(8): 2127-2143.
[2]. Tamási V, Hazai E, Porsmyr-Palmertz M, et al. GYKI-47261, a new AMPA [2-amino-3-(3-hydroxymethylisoxazole-4-yl)propionic acid] antagonist, is a CYP2E1 inducer. Drug Metab Dispos, 2003, 31(11): 1310-1314.
[3]. Bibbiani F, Oh JD, Kielaite A, et al. Combined blockade of AMPA and NMDA glutamate receptors reduces levodopa-induced motor complications in animal models of PD. Exp Neurol, 2005, 196(2): 422-429.
| Physical Appearance | Yellow solid |
| Storage | Store at -20°C |
| M.Wt | 395.71 |
| Cas No. | 220445-20-5 |
| Formula | C18H15ClN4·2HCl |
| Solubility | <35.92mg/ml in DMSO |
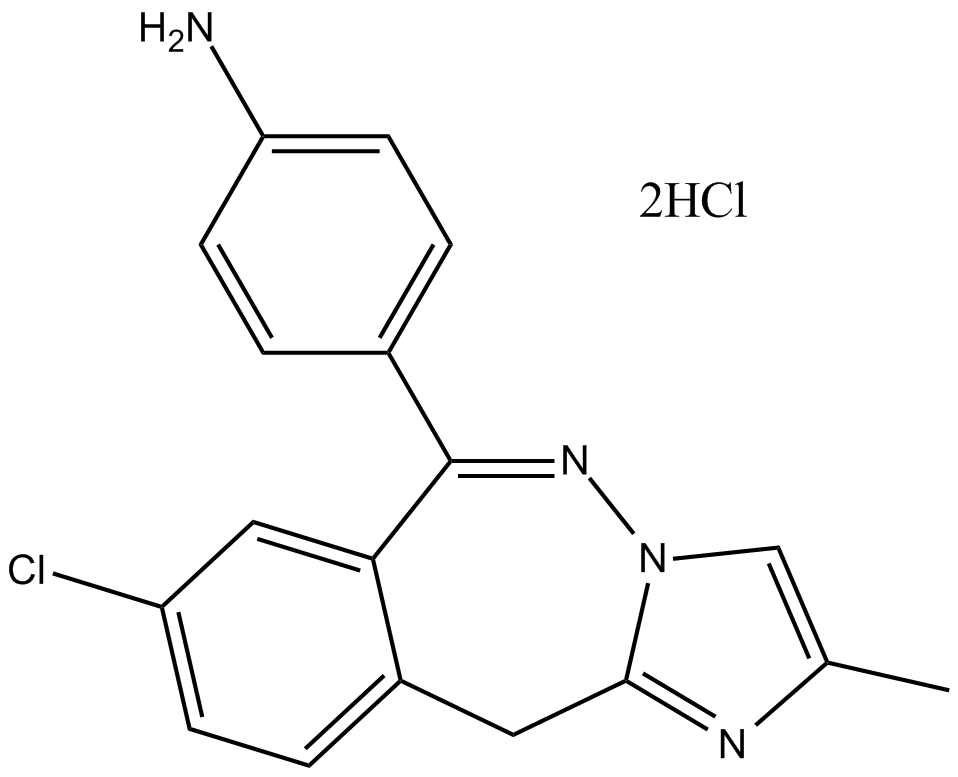
| Chemical Name | 4-(8-chloro-2-methyl-11H-benzo[e]imidazo[1,2-b][1,2]diazepin-6-yl)aniline dihydrochloride |
| SDF | Download SDF |
| Canonical SMILES | Cc(nc1C2)c[n]1N=C(c(cc1)ccc1N)c1c2ccc(Cl)c1.Cl.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure