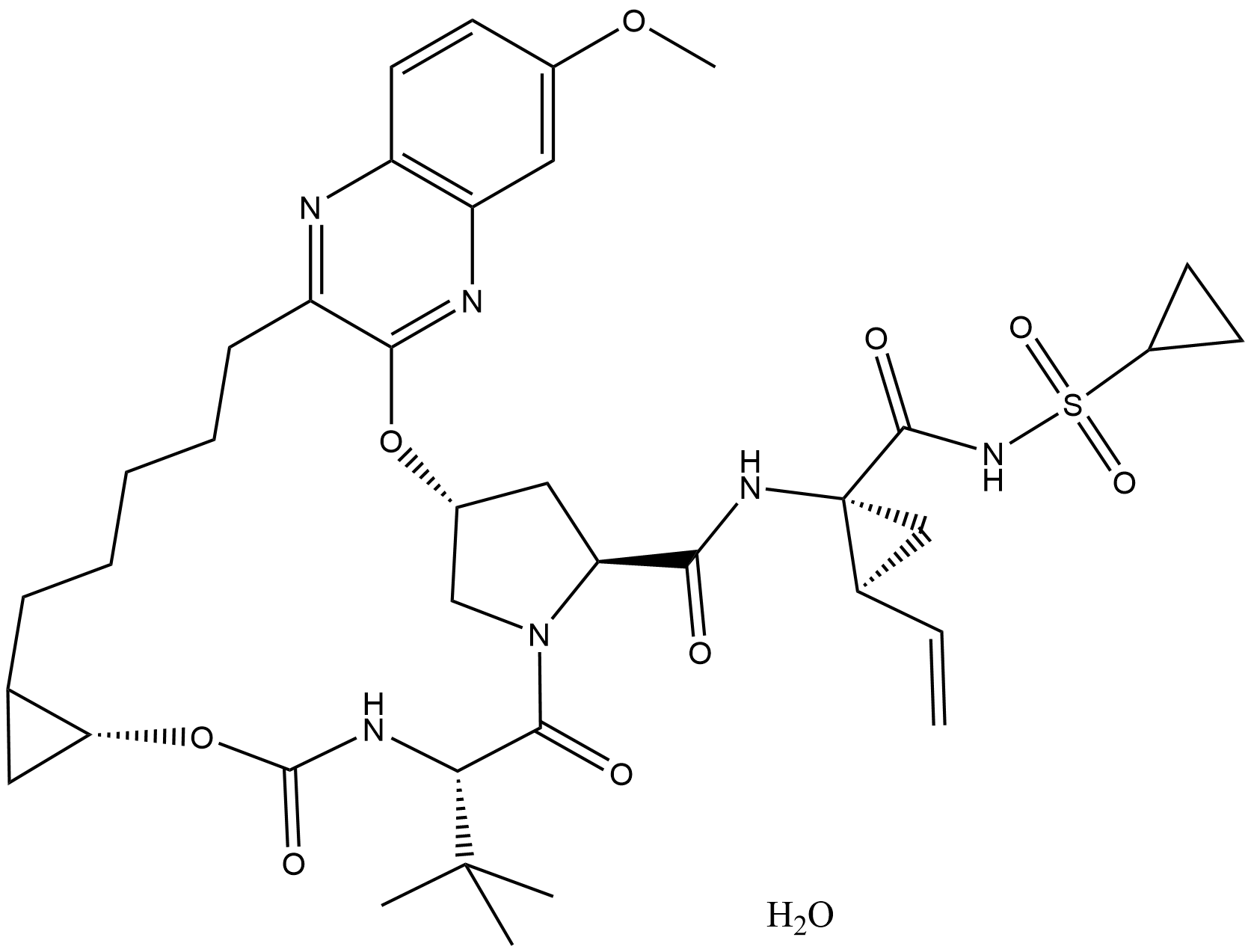
Grazoprevir hydrate
Grazoprevir hydrate (CAS No.: 1356960-17-6) is an oral direct-acting antiviral (DAA) with its core therapeutic target being the hepatitis C virus (HCV) NS3/4A protease. Its half-maximal effective concentrations (EC₅₀) against different HCV genotypes are as follows: GT1a (0.8 pmol/L), GT1b (0.3 pmol/L), GT4a (0.3 pmol/L), GT4b (0.16 pmol/L), and GT4g (0.24 pmol/L), with no clear IC₅₀ data available. The clinically effective therapeutic dose is 100 mg once daily (QD), and it is often formulated as a fixed-dose combination product (Zepatier) with the HCV NS5A inhibitor Elbasvir (CAS No.: 1370468-36-2, Catalog No.: A9068) at a dose of 50 mg QD. The treatment duration is adjusted to 8–16 weeks based on the patient’s genotype, baseline resistance-associated substitutions (RASs), and treatment history. Its biological activity is mainly reflected in specifically inhibiting the polyprotein cleavage mediated by the HCV NS3/4A protease, thereby blocking the viral replication cycle. It exhibits highly potent antiviral effects against HCV genotypes 1, 4, and 6, and is applicable to treatment-naive/treatment-experienced patients, those with compensated cirrhosis, HIV/HCV coinfected patients, patients with chronic kidney disease (CKD) stage 4–5 (including those on hemodialysis), and people who inject drugs. The sustained virologic response at 12 weeks (SVR12) rate ranges from 80% to 99%, with the best efficacy observed in GT1b patients (99%). For GT1a patients with baseline NS5A polymorphisms (such as M28 and Q30), combination therapy with ribavirin (RBV) and an extended treatment duration of 16 weeks are required. The drug is mainly metabolized by CYP3A, with a plasma protein binding rate > 98.8%, over 90% excreted in feces, and less than 1% excreted renally. No dosage adjustment is needed for patients with renal impairment, but co-administration with strong CYP3A inducers/inhibitors and OATP1B1/3 inhibitors should be avoided. It has a good safety profile; common adverse reactions include headache, fatigue, and nausea, with occasional elevation of alanine transaminase (ALT), which is mostly self-resolving.
References:
[1] [1] Vallet-Pichard A, Pol S. Grazoprevir/elbasvir combination therapy for HCV infection. Therap Adv Gastroenterol. 2017 Jan;10(1):155-167. doi: 10.1177/1756283X16671293. Epub 2016 Oct 17. PMID: 28286567; PMCID: PMC5330609.
[2] Wang SJ, Huang CF, Yu ML. Elbasvir and grazoprevir for the treatment of hepatitis C. Expert Rev Anti Infect Ther. 2021 Sep;19(9):1071-1081. doi: 10.1080/14787210.2021.1874351. Epub 2021 Jan 17. PMID: 33428488.
| Storage | Store at 4°C |
| M.Wt | 784.93 |
| Cas No. | 1350462-55-3 |
| Formula | C38H52N6O10S |
| Synonyms | MK-5172 hydrate |
| Solubility | Soluble in DMSO |
| Chemical Name | (33R,35S,91R,5S)-5-(tert-butyl)-N-((1R,2S)-1-((cyclopropylsulfonyl)carbamoyl)-2-vinylcyclopropyl)-17-methoxy-4,7-dioxo-2,8-dioxa-6-aza-1(2,3)-quinoxalina-3(3,1)-pyrrolidina-9(1,2)-cyclopropanacyclotetradecaphane-35-carboxamide hydrate |
| SDF | Download SDF |
| Canonical SMILES | COC1=CC2=C(N=C(CCCCCC3C[C@H]3OC4=O)C(O[C@H]5CN(C([C@H](C(C)(C)C)N4)=O)[C@H](C(N[C@@]([C@@H]6C=C)(C6)C(NS(C7CC7)(=O)=O)=O)=O)C5)=N2)C=C1.O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |