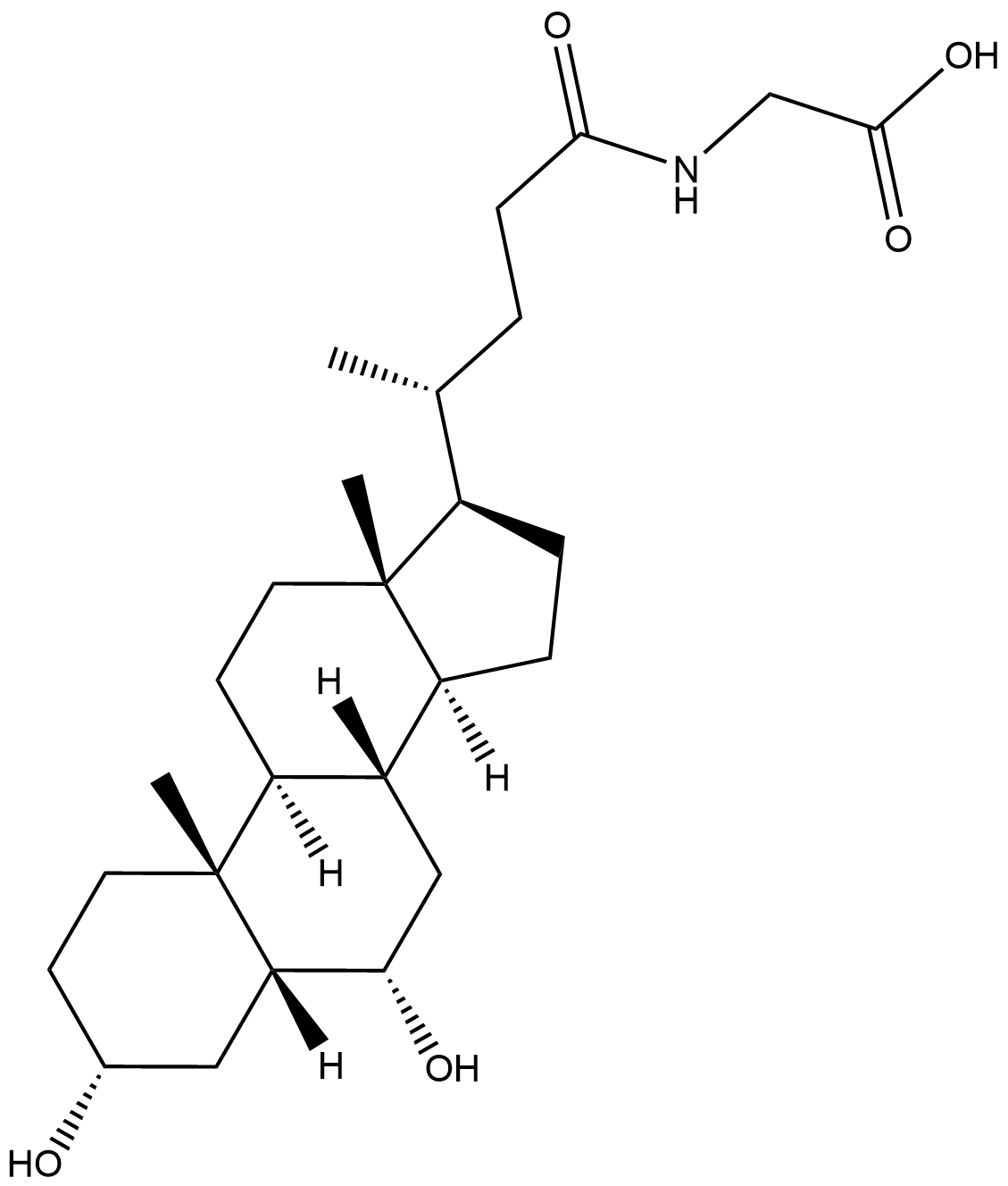
Glycohyodeoxycholic acid
Glycohyodeoxycholic acid is the main metabolite of Hyodeoxycholic acid in the human body. It is formed by the combination of hyodeoxycholic acid and glycine in the liver through amide bonds and belongs to secondary conjugated bile acids. Studies have shown that Glycohyodeoxycholic acid has a definite preventive effect on the formation of gallstones: by regulating the composition of the bile acid pool, it reduces the saturation of cholesterol in bile and inhibits the precipitation of cholesterol crystals. At the same time, it can activate the farnicol X receptor (FXR), promote the negative feedback regulation of bile acid synthesis and the excretion of cholesterol into bile, and optimize the lipid metabolism balance in the hepatointestinal circulation. As an endogenous metabolite, it is synthesized in the liver and secreted into the intestine with bile, participating in lipid digestion and absorption. Abnormal levels may reflect metabolic disorders in the liver and gallbladder or imbalance of intestinal flora, providing an important target for the prevention and treatment of gallstones and the research of bile acid metabolic-related diseases.
| Physical Appearance | A solid |
| Storage | Store at -20°C for 3 years. |
| M.Wt | 449.62 |
| Cas No. | 13042-33-6 |
| Formula | C26H43NO5 |
| Solubility | ≥51.1 mg/mL in DMSO; ≥24.95 mg/mL in EtOH; insoluble in H2O |
| Chemical Name | ((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoyl)glycine |
| SDF | Download SDF |
| Canonical SMILES | C[C@H](CCC(NCC(O)=O)=O)[C@H]1CC[C@@]2([H])[C@]3([H])C[C@H](O)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@@]21C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure