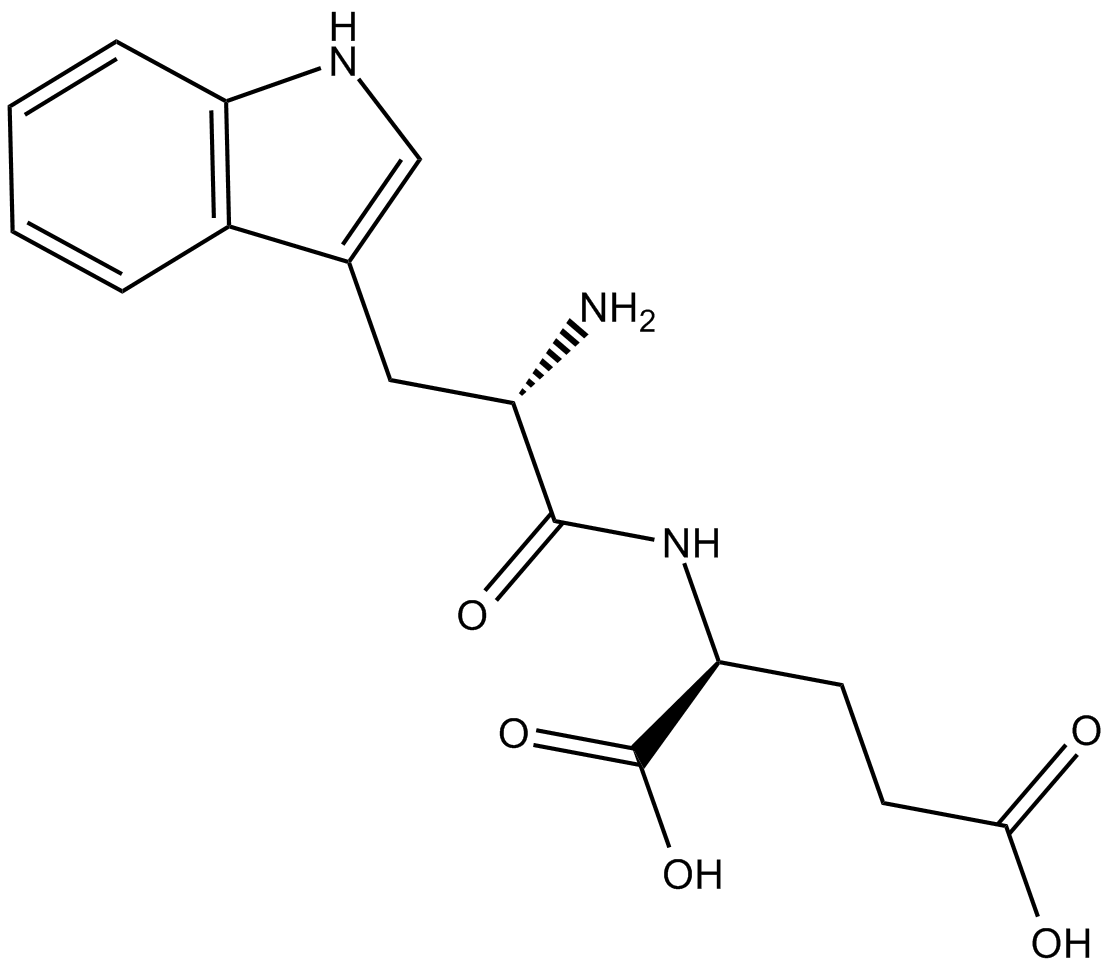
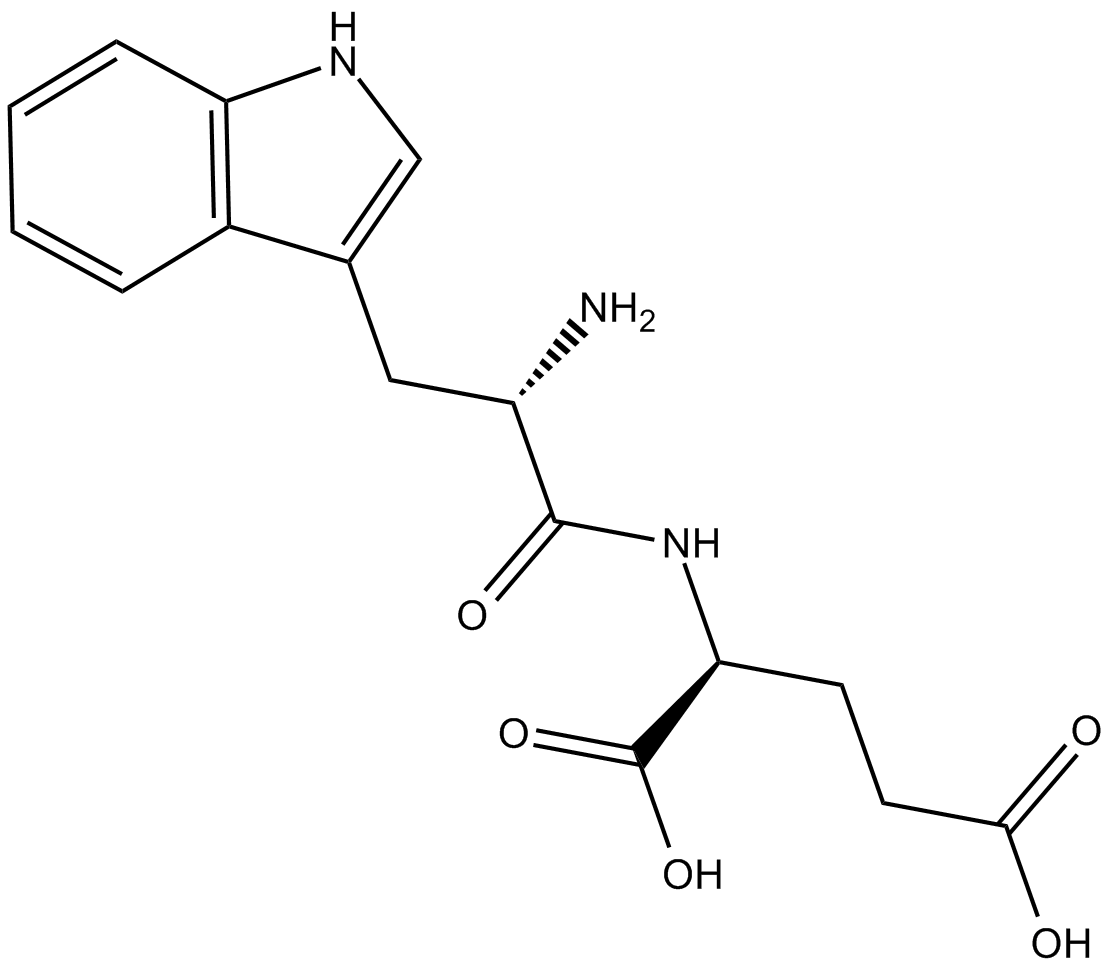
G3335
Kd = 8.34 μM
G3335 is a PPARγ antagonist.
The peroxisome proliferator-activated receptor gamma (PPARgamma) is a key therapeutic drug target for several conditions, such as inflammation, diabetes, hypertension, dyslipidemia, and cancer.
In vitro: Biacore 3000 study based on the surface plasmon resonance technique found that G3335 exhibited a highly specific binding affinity against PPARgamma and was able to block rosiglitazone, a potent PPARgamma agonist, in the stimulation of the interaction between the PPARgamma ligand-binding domain (LBD) and RXRalpha-LBD. Moreover, the yeast two-hybrid assays indicated that G3335 had strong antagonistic activity in perturbing rosiglitazone in the promotion of the PPARgamma-LBD-CBP interaction. In addition, G3335 could competitively bind to PPARgamma against 0.1 microM rosiglitazone to repress reporter-gene expression [1].
In vivo: In a previous study, the effect of rosiglitazone was examined on spinal cord injury (SCI) in rats. The animals were randomly divided into vehicle group, rosiglitazone treated group, and G3335 treated group. Locomotor function recovery was evaluated. Results showed that compared with the vehicle groups, the rosiglitazone could significantly ameliorate locomotor recovery, reduce NF-κB expression, and increase the proliferation of endogenous NPCs. In addition, when the PPAR-γ antagonist G3335 was applied, such effects were abolished [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Ye, F. ,Zhang, Z.S.,Luo, H.B., et al. The dipeptide H-Trp-Glu-OH shows highly antagonistic activity against PPARγ: Bioassay with molecular modeling simulation. ChemBioChem 7, 74-82 (2006).
[2] Meng, Q. Q.,Liang, X.J.,Wang, P., et al. Rosiglitazone enhances the proliferation of neural progenitor cells and inhibits inflammation response after spinal cord injury. Neuroscience Letters 503, 191-195 (2011).
| Storage | Store at -20°C |
| M.Wt | 333.3 |
| Cas No. | 36099-95-3 |
| Formula | C16H19N3O5 |
| Solubility | insoluble in EtOH; ≥14.35 mg/mL in DMSO; ≥5.76 mg/mL in H2O with ultrasonic |
| Chemical Name | L-tryptophyl-L-glutamic acid |
| SDF | Download SDF |
| Canonical SMILES | N[C@@H](Cc1c[nH]c2c1cccc2)C(N[C@@H](CCC(O)=O)C(O)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure