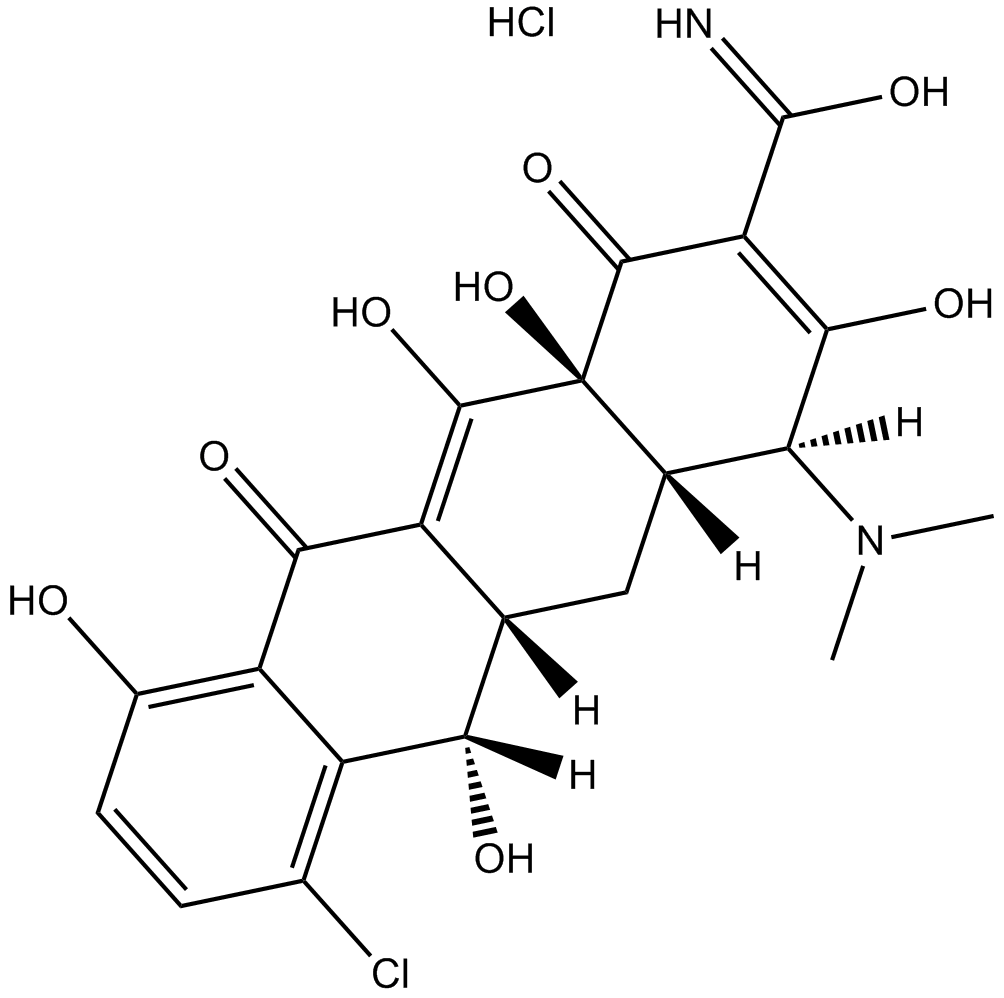
Demeclocycline hydrochloride
Demeclocycline hydrochloride (CAS No. 64-73-3) is a semisynthetic tetracycline compound, whose core targets include bacterial ribosomes (exerting antibacterial effects by inhibiting protein synthesis) and arginine vasopressin (ADH) receptors (blocking ADH-mediated water reabsorption); it can also regulate the expression of genes such as DDIT4 and FZD5 in brain tumor-initiating cells (BTICs) and activate monocytes. It is effective against most Gram-negative bacteria with an antibacterial spectrum similar to that of tetracycline. The concentrations commonly used in laboratory assays range from 1 to 10 μM (1, 5, and 10 μM for BTIC growth inhibition assays, and 10 μM for monocyte activation assays). Clinically effective therapeutic concentrations are achieved with the following oral dosages: 600–1200 mg per day for adults (administered in 2–4 divided doses), adjusted to 250 mg once or twice daily for patients with renal impairment; the drug concentration in breast milk ranges from 50 mcg/L to 1 mg/L, with acceptable safety for short-term use during lactation. Its biological activities are manifested as broad-spectrum antibacterial effects, induction of nephrogenic diabetes insipidus for the treatment of syndrome of inappropriate antidiuretic hormone secretion (SIADH)-associated hyponatremia, and inhibition of BTIC growth either directly or via monocyte activation. It has a serum protein binding rate of approximately 70%, is mainly eliminated via the renal pathway, and has an elimination half-life of about 1 hour. Common adverse reactions include gastrointestinal discomfort and nephrotoxicity, necessitating monitoring of liver and renal functions during long-term administration.
References:
[1] Miell J, Dhanjal P, Jamookeeah C. Evidence for the use of demeclocycline in the treatment of hyponatraemia secondary to SIADH: a systematic review. Int J Clin Pract. 2015 Dec;69(12):1396-417. doi: 10.1111/ijcp.12713. Epub 2015 Aug 19. PMID: 26289137; PMCID: PMC5042094.
[2] Sarkar S, Li Y, Mirzaei R, Rawji KS, Poon CC, Wang J, Kumar M, Bose P, Yong VW. Demeclocycline Reduces the Growth of Human Brain Tumor-Initiating Cells: Direct Activity and Through Monocytes. Front Immunol. 2020 Feb 21;11:272. doi: 10.3389/fimmu.2020.00272. PMID: 32153581; PMCID: PMC7047330.
[3] LiverTox: Clinical and Research Information on Drug Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Demeclocycline.
[4] Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Demeclocycline.
| Storage | Store at -20°C |
| M.Wt | 501.31 |
| Cas No. | 64-73-3 |
| Formula | C21H22Cl2N2O8 |
| Solubility | ≥12.93 mg/mL in DMSO; ≥2.42 mg/mL in EtOH gentle warming and ultrasonic; ≥16.63 mg/mL in H2O |
| Chemical Name | (4S,4aS,5aS,6S,12aS)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carbimidic acid hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | CN([C@@]1([H])[C@]2([H])C[C@@]([C@@](O)([H])C3=C(Cl)C=CC(O)=C3C4=O)([H])C4=C(O)[C@]2(O)C(C(C(O)=N)=C1O)=O)C.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |