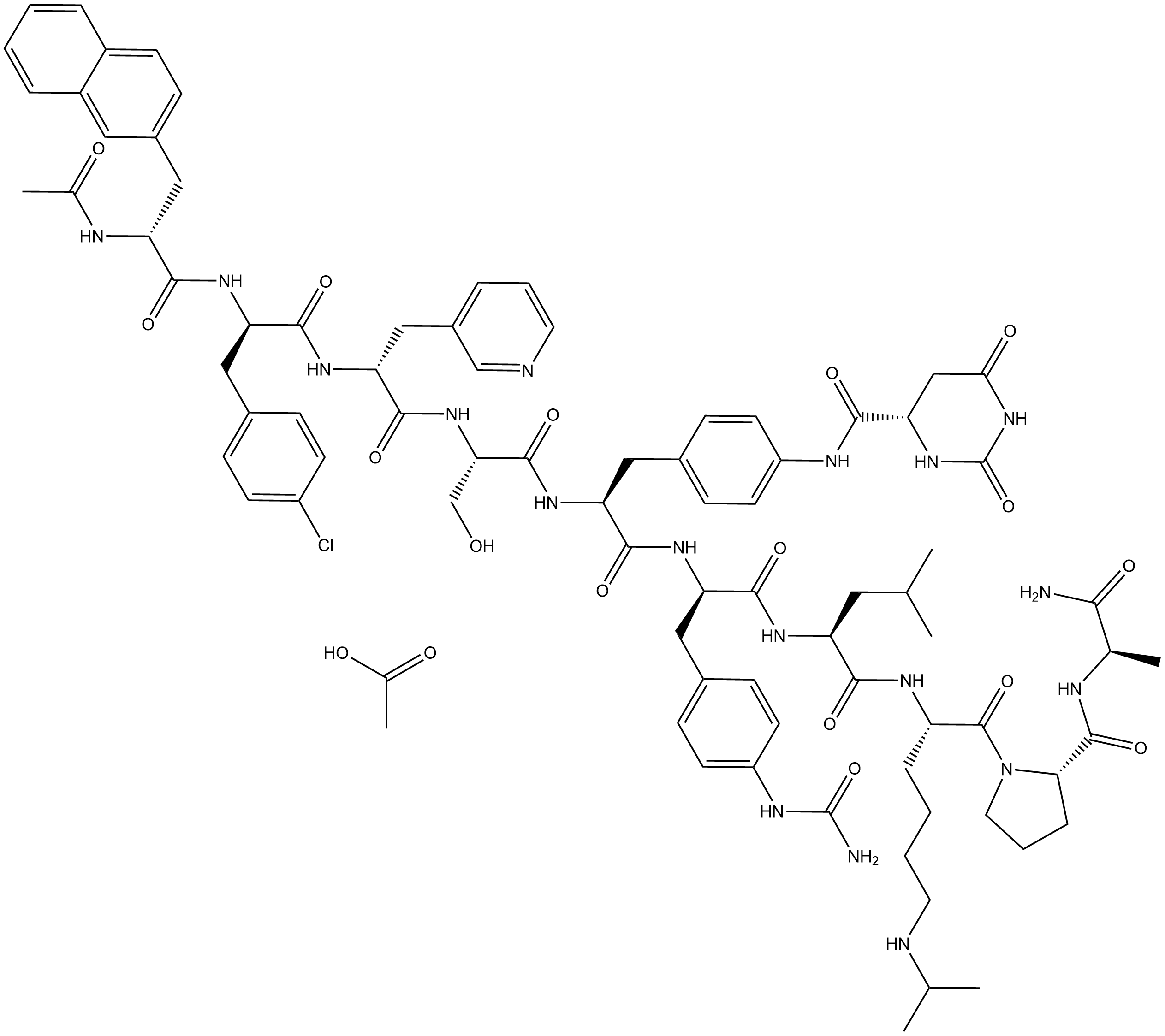
Degarelix acetate
Degarelix acetate (CAS No. 934016-19-0) is the acetate salt form of Degarelix (CAS No. 214766-78-6; catalog No.: BA2748). Degarelix is a potent and highly selective gonadotropin-releasing hormone (GnRH) receptor antagonist. Its primary biological activity is mediated through competitive binding to the GnRH receptor, a G protein–coupled receptor (GPCR), thereby blocking GnRH‑induced signal transduction, inhibiting pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and consequently inducing a sustained reduction in serum testosterone levels. It is used for the treatment of advanced prostate cancer.
Degarelix exhibits receptor-specific activity, with an in vitro half-maximal inhibitory concentration (IC₅₀) for competitive binding to the human GnRH receptor of approximately 0.1–1 nM, indicative of high affinity. Commonly used concentrations include 0.1–100 nM in in vitro cell-based assays (e.g., pituitary cells, prostate cancer cell lines) for receptor-binding validation and hormone secretion inhibition studies. In animal studies, subcutaneous administration at 0.1–1 mg/kg in rats and 0.3–1 mg/kg in rhesus monkeys can significantly reduce serum LH, FSH, and testosterone levels within 24–48 hours.
Clinically effective exposure corresponds to the approved dosing regimen for adult patients with advanced prostate cancer: an initial loading dose of 240 mg administered subcutaneously on Day 1 (given as two 120 mg injections), followed by a maintenance dose of 80 mg subcutaneously every 4 weeks. At steady state, this regimen maintains testosterone at castration levels (<0.5 ng/mL). Dose adjustment according to age or body weight is not required (with the exception of patients with severe hepatic or renal impairment). Degarelix is generally well tolerated; the most common adverse reactions include injection-site reactions and hot flashes.
References:
[1] Samant MP, Miller C, Hong DJ, Koerber SC, Croston G, Rivier CL, Rivier JE. Synthesis and biological activity of GnRH antagonists modified at position 3 with 3-(2-methoxy-5-pyridyl)-alanine. J Pept Res. 2005 Feb;65(2):284-91. doi: 10.1111/j.1399-3011.2005.00219.x. PMID: 15705170.
[2] Samant MP, Gulyas J, Hong DJ, Croston G, Rivier C, Rivier J. Iterative approach to the discovery of novel degarelix analogues: substitutions at positions 3, 7, and 8. Part II. J Med Chem. 2005 Jul 28;48(15):4851-60. doi: 10.1021/jm050134t. PMID: 16033265; PMCID: PMC2593149.
[3] Klotz L. Degarelix acetate for the treatment of prostate cancer. Drugs Today (Barc). 2009 Oct;45(10):725-30. doi: 10.1358/dot.2009.45.10.1417873. PMID: 20069136.
[4] Kawate N, Kanuki R, Hannan MA, Weerakoon WWPN. Inhibitory effects of long-term repeated treatments of a sustainable GnRH antagonist, degarelix acetate, on caprine testicular functions. J Reprod Dev. 2020 Dec 22;66(6):587-592. doi: 10.1262/jrd.2020-035. Epub 2020 Aug 21. PMID: 32830151; PMCID: PMC7768175.
[5] Huang Y, Chen J, Liu B, Wang H, Zhang L, Chen Z, Zhang Y. An efficient synthesis of deuterium-labeled degarelix acetate, a third-generation gonadotropin-releasing hormone receptor antagonist. J Labelled Comp Radiopharm. 2018 Apr;61(4):355-361. doi: 10.1002/jlcr.3567. Epub 2018 Mar 15. PMID: 28960413.
[6] Hjalte J, Hossain S, Hugerth A, Sjögren H, Wahlgren M, Larsson P, Lundberg D. Aggregation Behavior of Structurally Similar Therapeutic Peptides Investigated by 1H NMR and All-Atom Molecular Dynamics Simulations. Mol Pharm. 2022 Mar 7;19(3):904-917. doi: 10.1021/acs.molpharmaceut.1c00883. Epub 2022 Feb 1. PMID: 35104408; PMCID: PMC8905580.
[7] Kamada S, Sakamoto S, Kinoshita R, Zhao X, Kamasako T, Yamase R, Junryo R, Saito S, Sangjon P, Takei A, Yamada Y, Goto Y, Imamura Y, Iguchi T, Mizokami A, Suzuki H, Akakura K, Ichikawa T. Testosterone bounce predicts favorable prognoses for prostate cancer patients treated with degarelix. Prostate. 2024 May;84(7):636-643. doi: 10.1002/pros.24679. Epub 2024 Feb 27. PMID: 38413843.
[8] Kambe T, Yamasaki T, Yamamoto A, Nagoshi A, Fujiwara T, Mine Y, Hagimoto H, Igarashi A, Kokubun H, Murata S, Akagi N, Hattori Y, Abe Y, Tsutsumi N, Shibasaki N, Kawakita M. Dose compliance of estramustine phosphate in neoadjuvant chemohormonal therapy combined with degarelix acetate predicts the biochemical recurrence in patients with very high-risk prostate cancer who underwent robot-assisted radical prostatectomy. Int J Urol. 2024 Dec;31(12):1400-1407. doi: 10.1111/iju.15579. Epub 2024 Sep 10. PMID: 39253858.
| Storage | Store at -20°C sealed and dried. |
| M.Wt | 1692.34 |
| Cas No. | 934016-19-0 |
| Formula | C84H107ClN18O18 |
| Synonyms | FE 200486 |
| Solubility | ≥50.2 mg/mL in DMSO; ≥2.45 mg/mL in EtOH with ultrasonic; ≥17.07 mg/mL in H2O |
| Chemical Name | (S)-N-(4-((2S,5S,8R,11R,14R)-2-(((R)-1-(((S)-1-(((S)-1-((S)-2-(((R)-1-amino-1-oxopropan-2-yl)carbamoyl)pyrrolidin-1-yl)-6-(isopropylamino)-1-oxohexan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-1-oxo-3-(4-ureidophenyl)propan-2-yl)carbamoyl)-11-(4-chlorob |
| SDF | Download SDF |
| Canonical SMILES | CC(N[C@H](CC1=CC=C(C=CC=C2)C2=C1)C(N[C@H](CC3=CC=C(Cl)C=C3)C(N[C@H](CC4=CC=CN=C4)C(N[C@@H](CO)C(N[C@@H](CC5=CC=C(NC([C@H]6NC(NC(C6)=O)=O)=O)C=C5)C(N[C@H](CC7=CC=C(NC(N)=O)C=C7)C(N[C@@H](CC(C)C)C(N[C@@H](CCCCNC(C)C)C(N8CCC[C@H]8C(N[C@@H](C(N)=O)C)=O)=O)=O) |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |