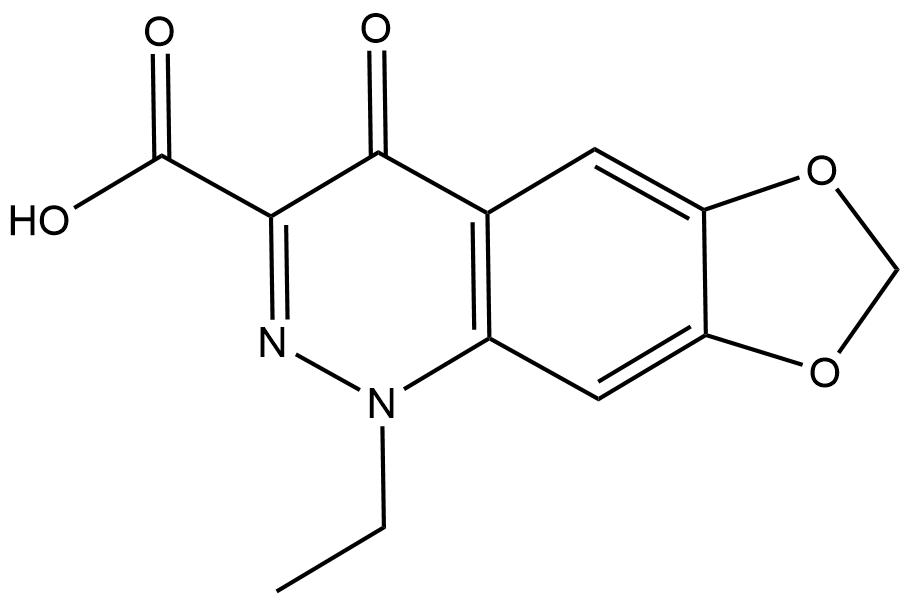
Cinoxacin
Cinoxacin (CAS No. 28657-80-9) is a synthetic organic acid antibiotic whose core target is the bacterial DNA synthesis pathway; its mechanism of action, similar to that of nalidixic acid, relies on inhibiting bacterial DNA replication, and its antibacterial activity is typically characterized by the minimum inhibitory concentration (MIC). Its in vitro MIC ranges from 2 to 8 μg/ml, effective against most Gram-negative bacteria such as Escherichia coli, Proteus mirabilis and indole-positive Proteus species; most strains of Klebsiella, Enterobacter and Serratia marcescens are susceptible to concentrations below 8 μg/ml, whereas Pseudomonas aeruginosa and Gram-positive bacteria remain resistant to concentrations under 64 μg/ml. The concentrations commonly used in laboratory assays are 1–256 μg/ml (for agar/broth dilution methods), and 30 μg per disk is standard for the disk diffusion method. Clinically effective therapeutic concentrations are achieved with the following oral dosages: 500 mg twice daily for adults with normal renal function, 250 mg twice daily for those with mild to moderate renal impairment, and 250 mg once daily for patients with severe renal impairment. Urinary concentrations reach the therapeutic level within 2 hours after oral administration, peak at 4–6 hours, and remain above the MIC of most Gram-negative uropathogens even at 12 hours post-dose. Cinoxacin exerts bactericidal effects, reducing bacterial colony counts by 3 log₁₀ at an inoculum of 5×10⁶ cfu/ml; it shows cross-resistance with nalidixic acid and oxolinic acid, and bacterial resistance is prone to induction upon serial subculture. It has a serum protein binding rate of approximately 70%, is mainly eliminated via the renal pathway with 60% excreted as the unchanged drug, and has an elimination half-life of about 1 hour, which is prolonged in cases of renal impairment. Clinically, it is indicated for the treatment of initial and recurrent urinary tract infections caused by susceptible Gram-negative bacteria, with mild and infrequent adverse reactions predominantly including gastrointestinal discomfort, headache and dizziness.
References:
[1] Lumish RM, Norden CW. Cinoxacin: in vitro antibacterial studies of a new synthetic organic acid. Antimicrob Agents Chemother. 1975 Feb;7(2):159-63. doi: 10.1128/AAC.7.2.159. PMID: 1094949; PMCID: PMC429096.
[2] Scavone JM, Gleckman RA, Fraser DG. Cinoxacin: mechanism of action, spectrum of activity, pharmacokinetics, adverse reactions, and therapeutic indications. Pharmacotherapy. 1982 Sep-Oct;2(5):266-72. doi: 10.1002/j.1875-9114.1982.tb03195.x. PMID: 6763208.
| Physical Appearance | A solid |
| Storage | -20°C |
| M.Wt | 262.22 |
| Cas No. | 28657-80-9 |
| Formula | C12H10N2O5 |
| Synonyms | Compound 64716 |
| Solubility | ≥12.65 mg/mL in DMSO with ultrasonic; insoluble in EtOH; insoluble in H2O |
| Chemical Name | 1-ethyl-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | O=C1C2=C(C=C(OCO3)C3=C2)N(CC)N=C1C(O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |