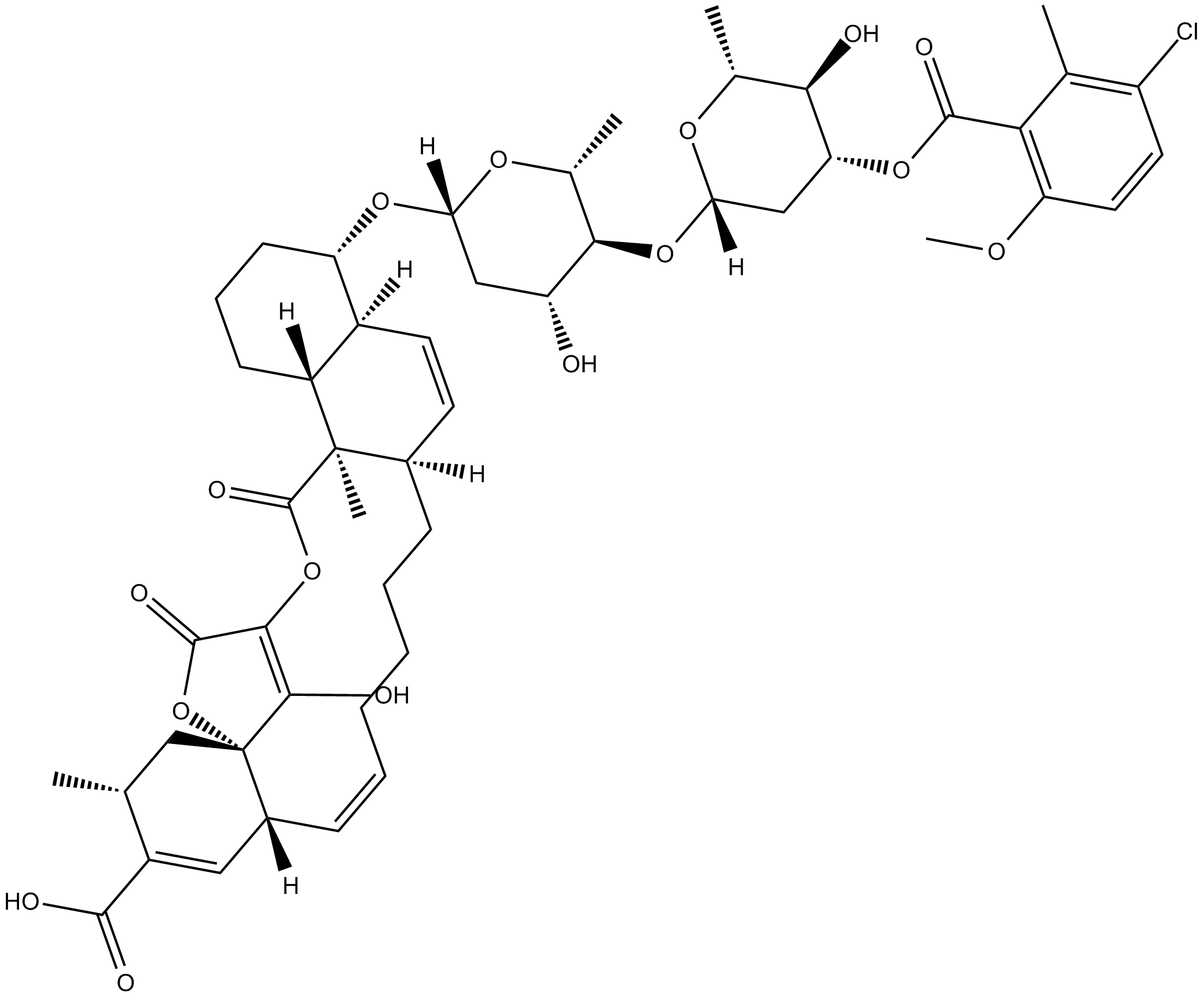
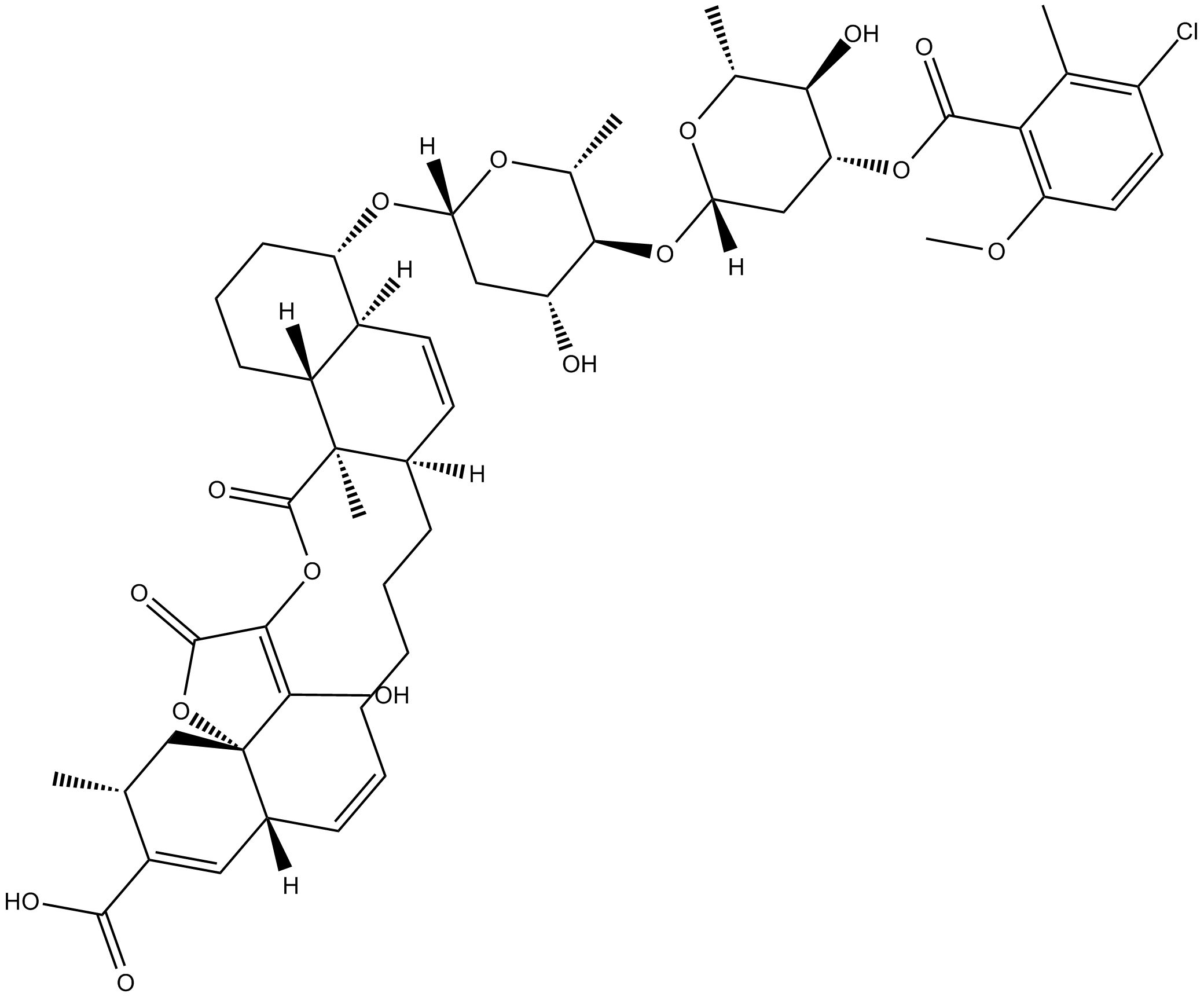
Chlorothricin
IC50: 173, 500, 260, and 120 μM for pyruvate carboxylases from Bacillus, Azotobacter, rat, and chicken, respectively.
Chlorothricin is a macrolide-type antibiotic.
Macrolides, a class of natural products belonging to the polyketide class of natural products, consist of a large macrocyclic lactone ring. The lactone rings are oftem 14-, 15-, or 16-membered. Some macrolides have been reported to have antibiotic or antifungal activity and are widely used as pharmaceutical drugs.
In vitro: In a previous study, chlorothricin was found to inhibit the reaction catalyzed by pyruvate carboxylase from Bacillus stearothermophilus. Moreover, with steady-state kinetic measurements, inhibition of the overall reaction was found to be competitive with the allosteric activator of this enzyme, CoASAc, and non-competitive with respect to both substrates of MgATP and pyruvate. These findings strongly indicated that the site 1conformation of pyruvate carboxylase responsible for the regulation of the overall enzyme activity could be influenced by chlorothricin and CoASAc in an antagonistic manner [1].
In vivo: Up to now, there is no animal in vivo data reported.
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1] Schindler PW, Zhner H. Mode of action of the macrolide-type antibiotic, chlorothricin. Kinetic study of the inhibition of pyruvate carboxylase from Bacillus stearothermophilus. Eur J Biochem. 1973 Nov 15;39(2):591-600.
| Physical Appearance | A white solid |
| Storage | Store at -20°C |
| M.Wt | 955.5 |
| Cas No. | 34707-92-1 |
| Formula | C50H63ClO16 |
| Synonyms | Antibiotic K 818A |
| Solubility | Soluble in ethanol;Soluble in methanol;Soluble in DMSO;Soluble in dimethyl formamide |
| Chemical Name | (4S,4aS,6aR,11E,12aR,15R,16aS,21aR,21bR)-4-[[4-O-[3-O-(3-chloro-6-methoxy-2-methylbenzoyl)-2,6-dideoxy-β-D-arabino-hexopyranosyl]-2,6-dideoxy-β-D-arabino-hexopyranosyl]oxy]-1,2,3,4,4a,6a,7,8,9,10,12a,15,16,21,21a,21b-hexadecahydro-22-hydroxy-15,21a-dimeth |
| SDF | Download SDF |
| Canonical SMILES | OC([C@]1([C@@]([H])(C=C([C@@H](C1)C)C(O)=O)/C=C\CCCC[C@]2([H])C=C[C@@]3([H])[C@](CCC[C@@H]3O[C@@]([H])(C[C@H]4O)O[C@@H]([C@H]4O[C@@]([H])(C[C@H]5OC(C6=C(OC)C=CC(Cl)=C6C)=O)O[C@@H]([C@H]5O)C)C)([H])[C@@]27C)OC8=O)=C8OC7=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure