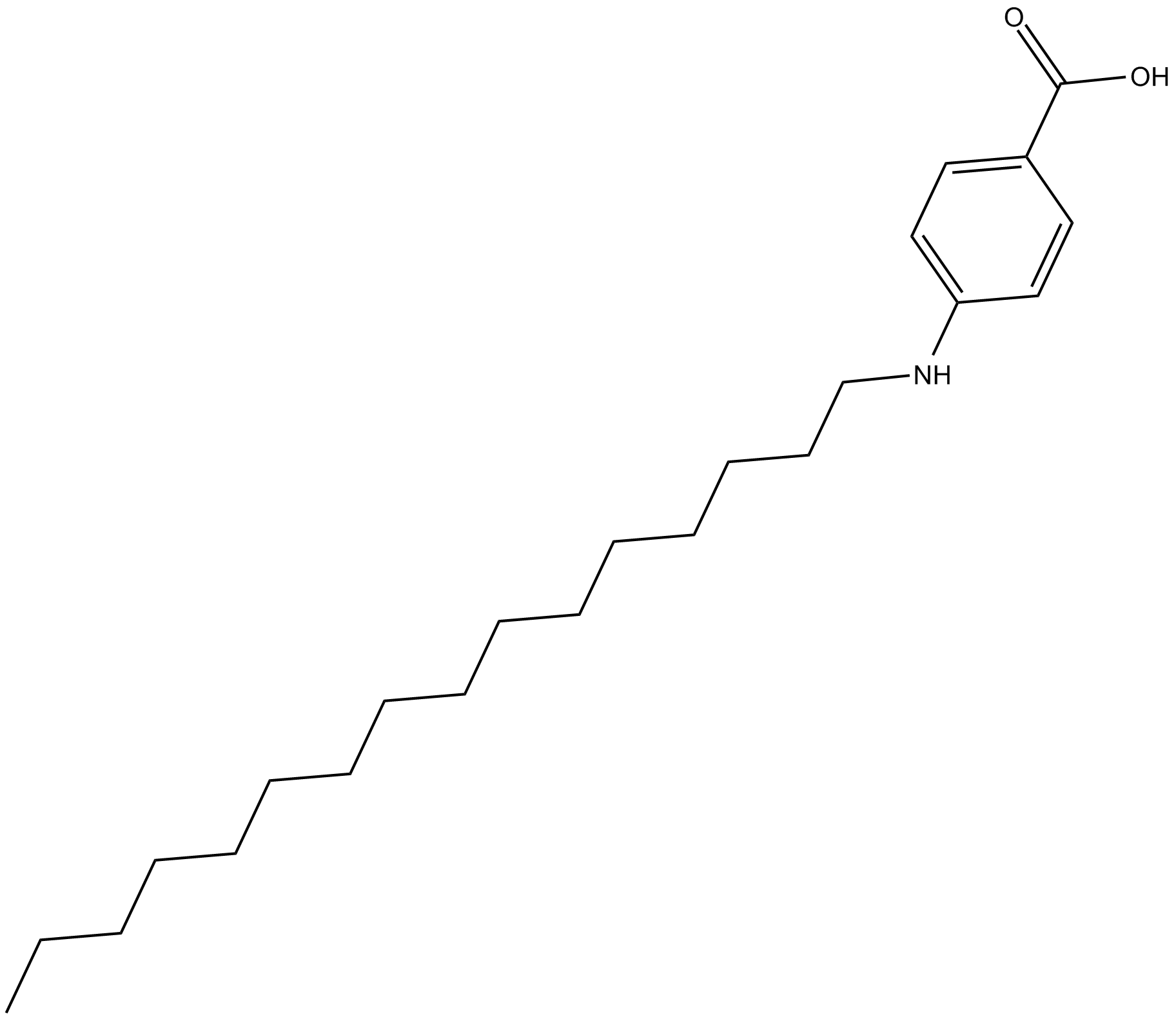
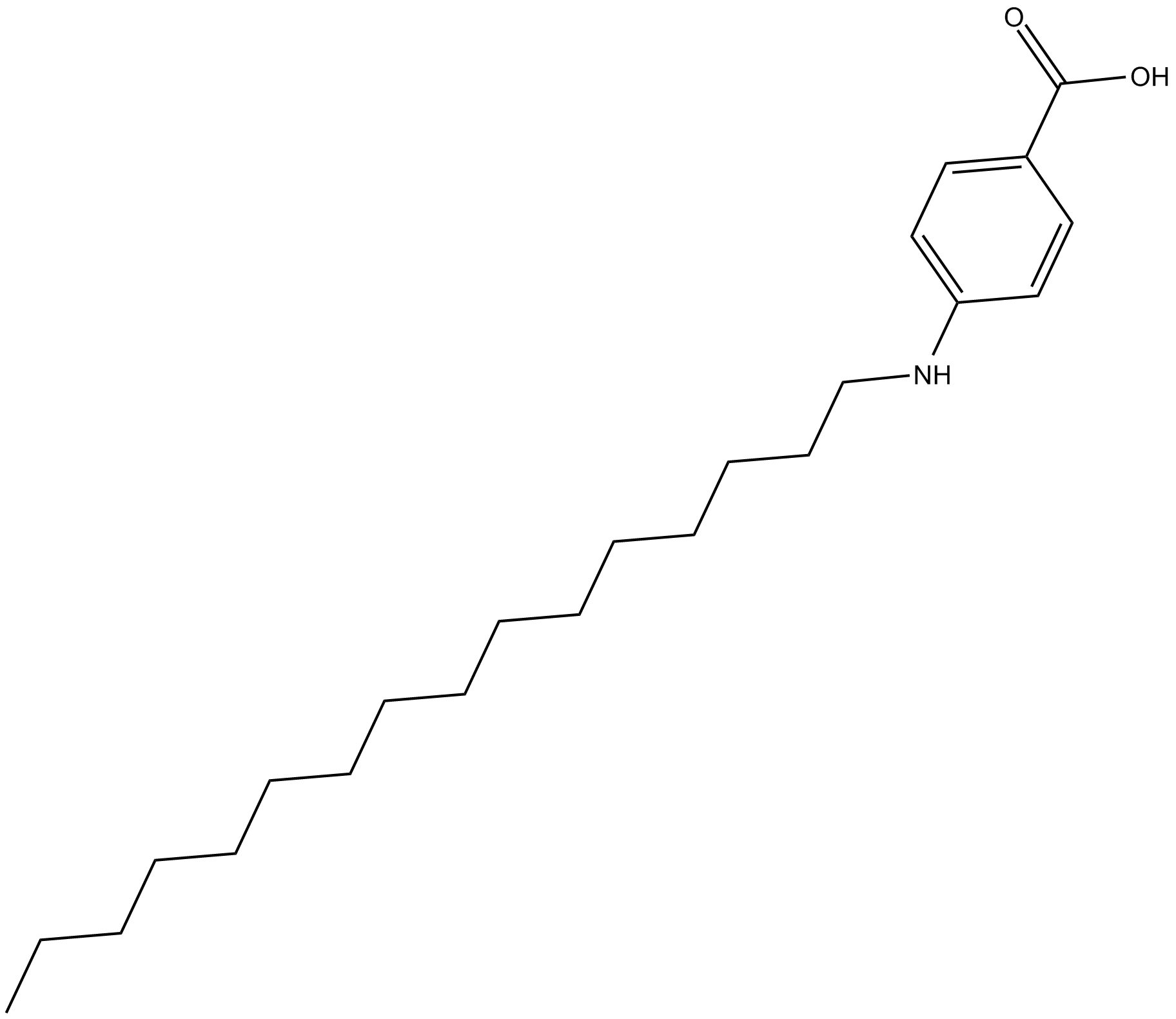
Cetaben
Cetaben is an unique PPARα-independent peroxisome proliferator.
The fibrate class of hypolipidemic drugs, such as fenofibrate and clofibrate, elicit their effects via binding to and activating peroxisome proliferator-activated receptor α (PPARα).
In vitro: Previous study showed that cetaben could cause little but reversible proliferation and morphological heterogeneity with the occurrence of dumbbell- and cup-shaped peroxisomal profiles. Peroxisomes in HepG2 cells showed marked variation in size and shape. Cetaben treatment of HepG2 cells was able to lead to disintegration of Golgi regions and augmented mitochondrial matrix [1].
In vivo: Animal study found that the changes in large scale of liver non-peroxisomal parameters were compared after 10 days administration of both cetaben and clofibric acid 200 mg/kg/day to male Wistar rats. No analogical changes were observed after cetaben treatment in the livers of experimental animals. It was also found that both drugs could increase the activities of alanine-glyoxylate aminotransferase-1 and acetylcarnitine transferase--enzymes with proven mitochondrial and peroxisomal location. Contrary to clofibric acid, cetaben did not increase solubilization of peroxisomal enzymes [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Kovacs, W. ,Walter, I., and Stangl, H. Cetaben-induced changes on the morphology and peroxisomal enzymes in MH1C1 rat hepatoma and HepG2 human hepatoblastoma cells. Histochemistry and Cell Biology 115, 509-519 (2001).
[2] Chandoga, J. ,Hampl, L.,Turecky, L., et al. Cetaben is an exceptional type of peroxisome proliferator. International Journal of Biochemistry 26(5), 679-696 (1994).
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 361.6 |
| Cas No. | 55986-43-1 |
| Formula | C23H39NO2 |
| Synonyms | Hexadecylamino-p-amino Benzoic Acid |
| Solubility | ≤1mg/ml in ethanol;20mg/ml in DMSO;20mg/ml in dimethyl formamide |
| Chemical Name | 4-(hexadecylamino)-benzoic acid |
| SDF | Download SDF |
| Canonical SMILES | CCCCCCCCCCCCCCCCNc(cc1)ccc1C(O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure