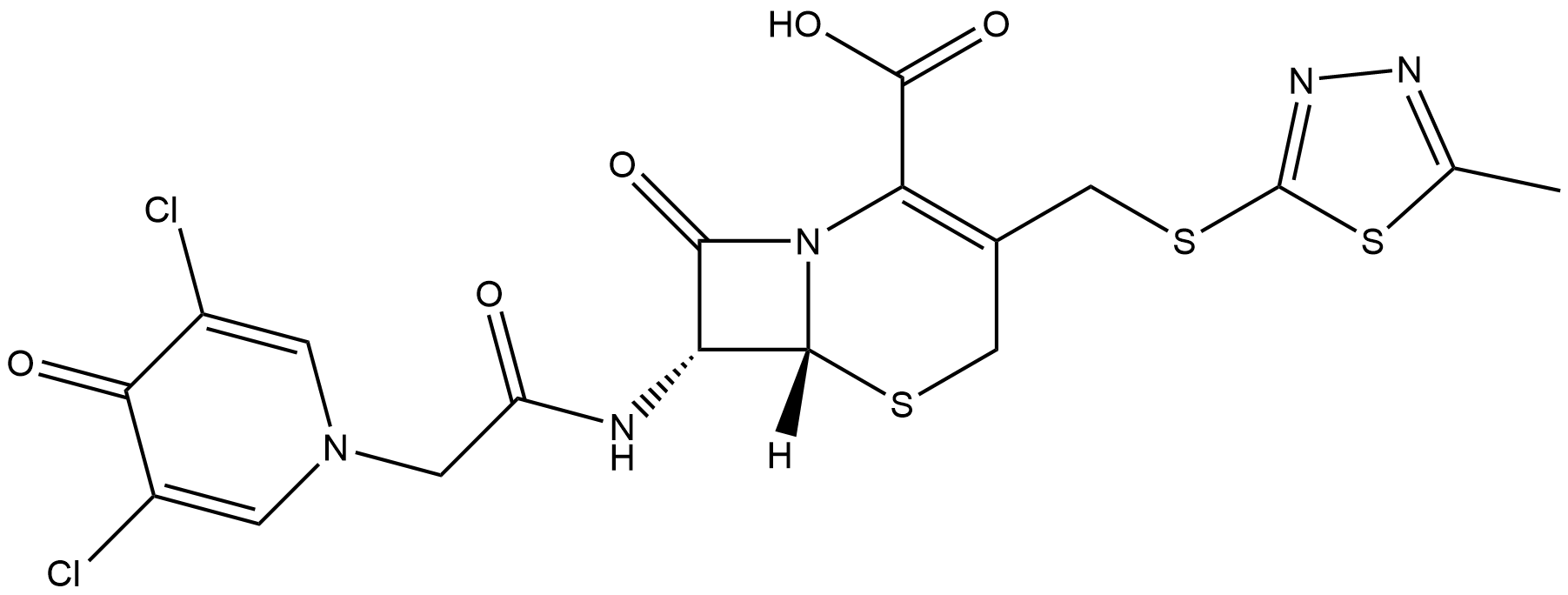
Cefazedone
Cefazedone (CAS No. 56187-47-4) is a first-generation cephalosporin broad-spectrum antibiotic. Its core bioactivity is inhibition of bacterial cell wall synthesis, and its target is bacterial penicillin-binding proteins (PBPs). It shows antibacterial activity against Gram-positive bacteria (such as Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecalis) and Gram-negative bacteria (such as Escherichia coli, Klebsiella spp., Haemophilus influenzae, Klebsiella pneumoniae), and its antibacterial effect is not affected by β-lactamase production. No definitive IC50/EC50 data are available (as a time-dependent antibiotic, the key PK/PD parameter is the time that free drug concentration exceeds the MIC, fT>MIC). MIC values are strain-specific. For clinical isolates (community-acquired pneumonia related), MIC is 0.25~1 mg/L. In vitro, the MIC range against Escherichia coli is 0.125~4 μg/ml (MIC50 0.25 μg/ml, MIC90 0.5 μg/ml), Enterococcus faecalis 4~64 μg/ml (MIC50 16 μg/ml, MIC90 32 μg/ml), oxacillin-resistant Staphylococcus aureus 8~64 μg/ml (MIC50 16 μg/ml, MIC90 64 μg/ml), Klebsiella spp. 0.125~4 μg/ml (MIC50 0.25 μg/ml, MIC90 1 μg/ml). Commonly used application concentrations: in vitro antibacterial testing uses a gradient of 0.125~1024 μg/ml (broth dilution method); in animal experiments (beagle dogs), the intravenous infusion dose is 32 mg/kg (20 min infusion), and there is no obvious pharmacokinetic interaction when combined with etimicin. Clinically effective therapeutic concentrations correspond to adult intravenous infusion regimens: for the treatment of community-acquired pneumonia, 2 g every 12 hours (30 min infusion), with a steady-state peak concentration of about 175.22±36.28 mg/L, AUC0-∞ 280.51±68.17 mg·h/L, protein binding rate 93%~96%, free drug fraction 4%~7%, and mean fT>MIC of 55.45±8.12% (needs to reach 40%~60% of the dosing interval to ensure efficacy). It is suitable for infections caused by susceptible bacteria in the respiratory tract, urinary tract, abdominal cavity, surgical sites, and skin and soft tissues.
References:
[1] Cullmann W, Opferkuch W, Stieglitz M, Werkmeister U. A comparison of the antibacterial activities of N-formimidoyl thienamycin (MK0787) with those of other recently developed beta-lactam derivatives. Antimicrob Agents Chemother. 1982 Aug;22(2):302-7. doi: 10.1128/AAC.22.2.302. PMID: 6821459; PMCID: PMC183729.
[2] Cui Y, Ma N, Li X, Lv C, Li M, Li M, Song L, Liu M, Li Q, Bi K. Development of an ultra fast liquid chromatography-tandem mass spectrometry method for simultaneous determination of cefazedone and etimicin in beagle dog plasma: Application to the pharmacokinetic study of the combination of cefazedone and etimicin injections. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Dec 15;973C:97-103. doi: 10.1016/j.jchromb.2014.10.018. Epub 2014 Oct 23. PMID: 25464101.
[3] Gao L, Zhu Y, Lyu Y, Hao FL, Zhang P, Wei MJ. A Pharmacokinetic and Pharmacodynamic Study on Intravenous Cefazedone Sodium in Patients with Community-acquired Pneumonia. Chin Med J (Engl). 2015 May 5;128(9):1160-4. doi: 10.4103/0366-6999.156086. PMID: 25947397; PMCID: PMC4831541.
| Physical Appearance | A solid |
| Storage | -20°C |
| M.Wt | 548.44 |
| Cas No. | 56187-47-4 |
| Formula | C18H15Cl2N5O5S3 |
| Synonyms | Refosporen |
| Solubility | ≥50 mg/mL in DMSO; insoluble in EtOH; insoluble in H2O |
| Chemical Name | (6R,7R)-7-(2-(3,5-dichloro-4-oxopyridin-1(4H)-yl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | O=C(O)C1=C(CSC2=NN=C(C)S2)CS[C@@]3([H])N1C([C@H]3NC(CN4C=C(Cl)C(C(Cl)=C4)=O)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |