Caspofungin
Caspofungin (CAS: 162808-62-0) is a small-molecule inhibitor of β-1,3-glucan synthase, an enzyme essential for the biosynthesis of β-(1,3)-D-glucan, a fundamental cell wall component in pathogenic fungi such as Candida albicans. Caspofungin selectively inhibits β-1,3-glucan synthase activity with a reported IC50 of approximately 0.6 nmol/L in membrane preparations of C. albicans. Caspofungin demonstrates potent antifungal activity against various Candida isolates, including azole-resistant strains (MIC90 ≤0.5 μg/mL), and shows prolonged post-antifungal effects lasting 6–8 hours. It is widely utilized in biomedical research involving fungal infections and antifungal therapeutics.
- 1. Trinh Phan-Canh, Sabrina Jenull, et al. "White-Brown switching controls phenotypic plasticity and virulence of Candida auris." Cell Rep. 2025 Jul 22;44(7):115976 PMID: 40638387
- 2. Christopher Zajac, Nancy E. Scott, et al. "Hotspot gene conversion between FKS1 and FKS2 in echinocandin resistant Candida glabrata serial isolates." NPJ Antimicrob Resist. 2025 Apr 17;3(1):31. PMID: 40247099
- 3. Pengju Yu, Mi Zhou, et al. "Targeted regulation of sterol biosynthesis genes according to perturbations in ergosterol biosynthesis in fungi." J Adv Res2025 Jan 30:S2090-1232(25)00065-7. PMID: 39892608
- 4. Dante G Calise, Sung Chul Park, et al. "An oxylipin signal confers protection against antifungal echinocandins in pathogenic aspergilli." Nat Commun. 2024 May 4;15(1):3770. PMID: 38704366
- 5. Bao Gia Vu, Lucia Simonicova, et al. "Calcineurin is required for Candida glabrata Pdr1 transcriptional activation." bioRxiv. July 10, 2023
- 6. Naemah Alkhars, Anthony Gaca, et al. "Antifungal Susceptibility of OralCandidaIsolates from Mother-Infant Dyads to Nystatin, Fluconazole, and Caspofungin." J Fungi (Basel). 2023 May 17;9(5):580. PMID: 37233291
- 7. Chengcheng Hu, Mi Zhou, et al. "Coordinated Regulation of Membrane Homeostasis and Drug Accumulation by Novel Kinase STK-17 in Response to Antifungal Azole Treatment." Microbiol Spectr. 2022 Feb 23;10(1):e0012722. PMID: 35196787
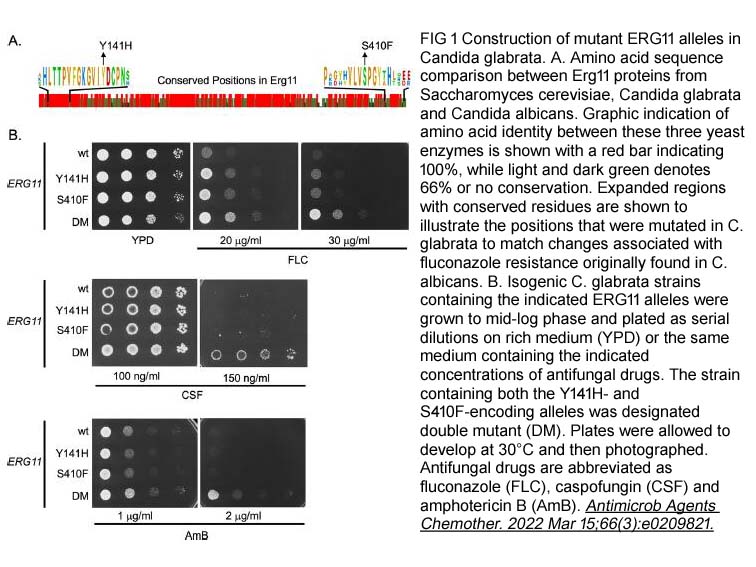
- 8. Bao Gia Vu, W. Scott Moye-Rowley. "Azole-resistant alleles ofERG11inCandida glabratatrigger activation of the Pdr1 and Upc2A transcription factors." Antimicrob Agents Chemother. 2022 Mar 15;66(3):e0209821. PMID: 35007132
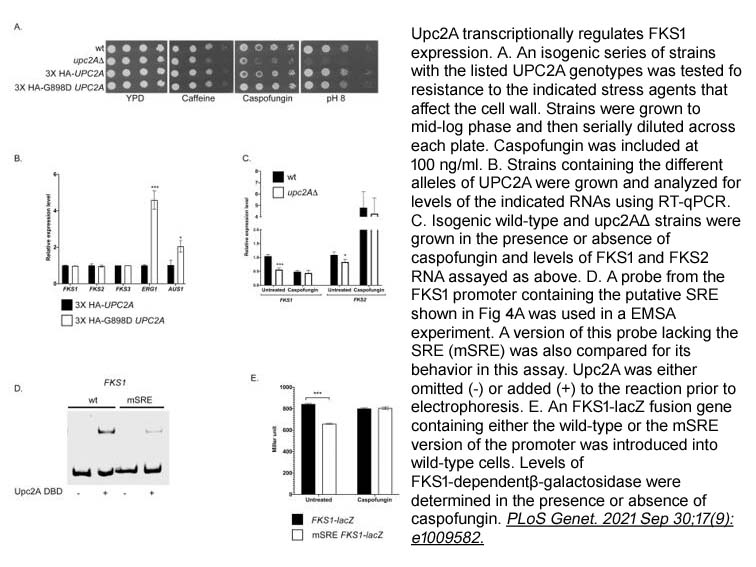
- 9. Bao Gia Vu, Mark A. Stamnes, et al. "The Candida glabrata Upc2A transcription factor is a global regulator of antifungal drug resistance pathways." PLoS Genet. 2021 Sep 30;17(9):e1009582. PMID: 34591857
- 10. Hoyer AR, Johnson CJ, et al. "Echinocandin Treatment of Candida albicans Biofilms Enhances Neutrophil Extracellular Trap Formation." Antimicrob Agents Chemother. 2018 Aug 27;62(9). pii: e00797-18. PMID: 29987146
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 1092.33 |
| Cas No. | 162808-62-0 |
| Formula | C53H89N9O15 |
| Solubility | ≥48.1 mg/mL in DMSO |
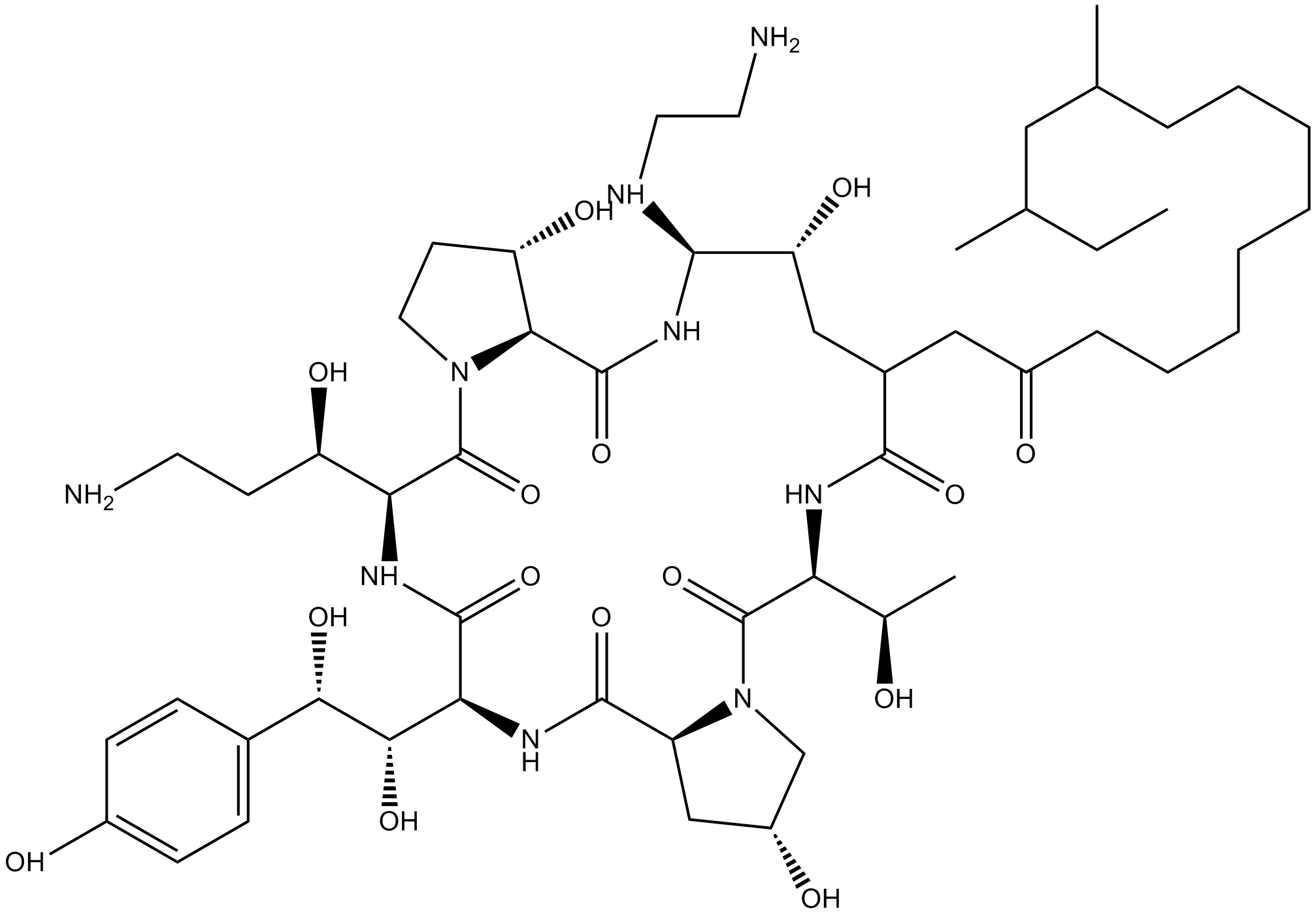
| Chemical Name | (2R,6S,11R,12R,14aS,15S,20S,23S,25aS)-20-((R)-3-amino-1-hydroxypropyl)-12-((2-aminoethyl)amino)-23-((1S,2S)-1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl)-9-(11,13-dimethyl-2-oxopentadecyl)-2,11,15-trihydroxy-6-((R)-1-hydroxyethyl)hexadecahydro-1H-dipyrrolo[2,1- |
| SDF | Download SDF |
| Canonical SMILES | O=C([C@](NC([C@](NC([C@@](C[C@H]1O)([H])N(C1)C2=O)=O)([H])[C@H](O)[C@H](C(C=C3)=CC=C3O)O)=O)([H])[C@H](O)CCN)N(CC[C@@H]4O)[C@]4([H])C(N[C@@H]([C@@H](C[C@@H](C(N[C@@]2([H])[C@H](O)C)=O)NC(CCCCCCCC[C@@H](C)C[C@@H](C)CC)=O)O)NCCN)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data

Related Biological Data
Related Biological Data