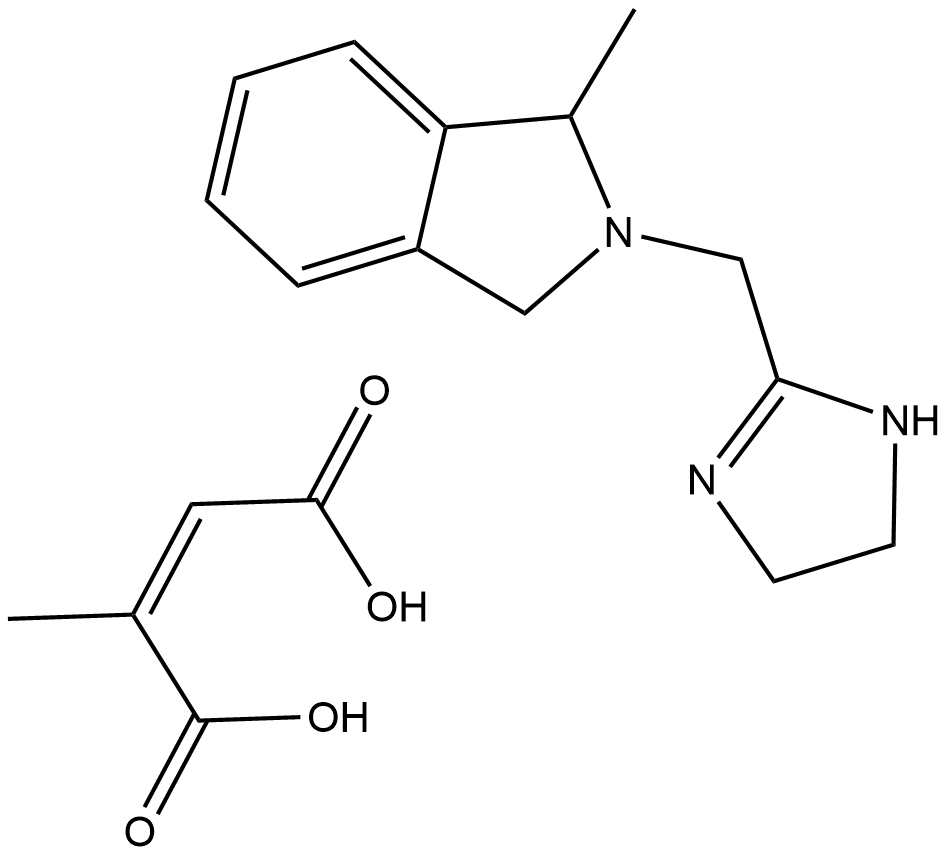
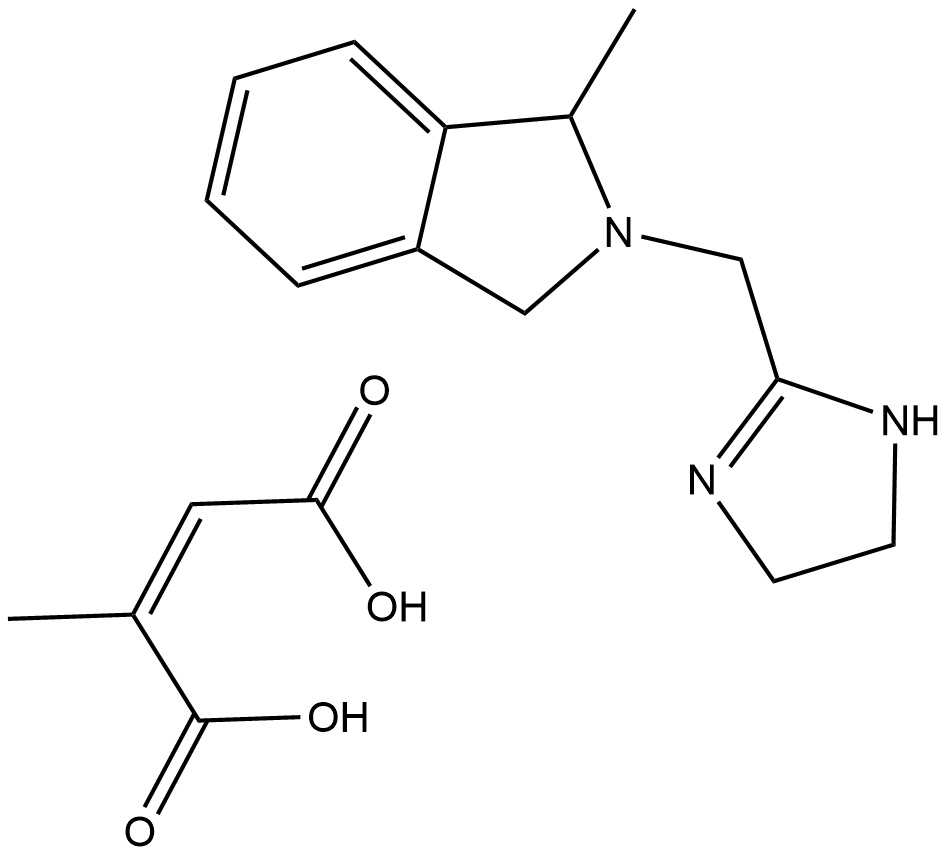
BRL 44408 (maleate)
BRL 44408 maleate is a selective antagonist of α2A-adrenoceptor with Ki values of 1.7 and 144.5 nM for α2A- and α2B-adrenoceptors, respectively.
α2A-adrenoceptor is a G-protein coupled receptor. α2-adrenergic receptors include three subtypes: α2A, α2B and α2C, which play an important role in regulating neurotransmitter release from adrenergic neurons in the central nervous system and from sympathetic nerves.
BRL 44408 maleate is a selective α2A-adrenoceptor antagonist. In rat brain, BRL 44408 inhibited the binding of 5-HTIA receptor radioligands 8-OH-DPAT and RX 821002 to cortical membranes with Ki values of 199 and 338 nM respectively, which suggested that BRL 44408 recognized 5-HT1A receptor [1].
In wild type and monoamine oxidase-A knockout (MAO-A KO) mice, BRL 44408 (100 nM) inhibited the effect of dexmedetomidine, the α2 agonist. BRL 44408 (100 nM) increased evoked noradrenaline (NA) efflux in MAO-A KO mice. In wild type mice, BRL 44408 antagonized the agonist effect of dexmedetomidine with pKB value of 7.75, which inhibited locus coeruleus (LC) cell firing with EC50 value of 2.6 nM [2]. In the forced swim test, BRL 44408 reduced immobility time. In schedule-induced polydipsia assay, BRL 44408 increased adjunctive water intake, which suggested that BRL 44408 exhibited antidepressant-like response. In a visceral pain model, BRL 44408 exhibited analgesic activity [3].
References:
[1]. Meana JJ, Callado LF, Pazos A, et al. The subtype-selective alpha 2-adrenoceptor antagonists BRL 44408 and ARC 239 also recognize 5-HT1A receptors in the rat brain. Eur J Pharmacol, 1996, 312(3): 385-388.
[2]. Owesson CA, Seif I, McLaughlin DP, et al. Different alpha(2) adrenoceptor subtypes control noradrenaline release and cell firing in the locus coeruleus of wildtype and monoamine oxidase-A knockout mice. Eur J Neurosci, 2003, 18(1): 34-42.
[3]. Dwyer JM, Platt BJ, Rizzo SJ, et al. Preclinical characterization of BRL 44408: antidepressant- and analgesic-like activity through selective alpha2A-adrenoceptor antagonism. Int J Neuropsychopharmacol, 2010, 13(9): 1193-1205.
| Physical Appearance | A crystalline solid |
| Storage | Desiccate at -20°C |
| M.Wt | 331.4 |
| Cas No. | 681806-46-2 |
| Formula | C13H17N3·C4H4O4 |
| Solubility | <33.14mg/ml in H2O |
| Chemical Name | 2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole, 2Z-butenedioate |
| SDF | Download SDF |
| Canonical SMILES | CC1N(CC2=NCCN2)Cc2c1cccc2.C/C(\C(O)=O)=C/C(O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure