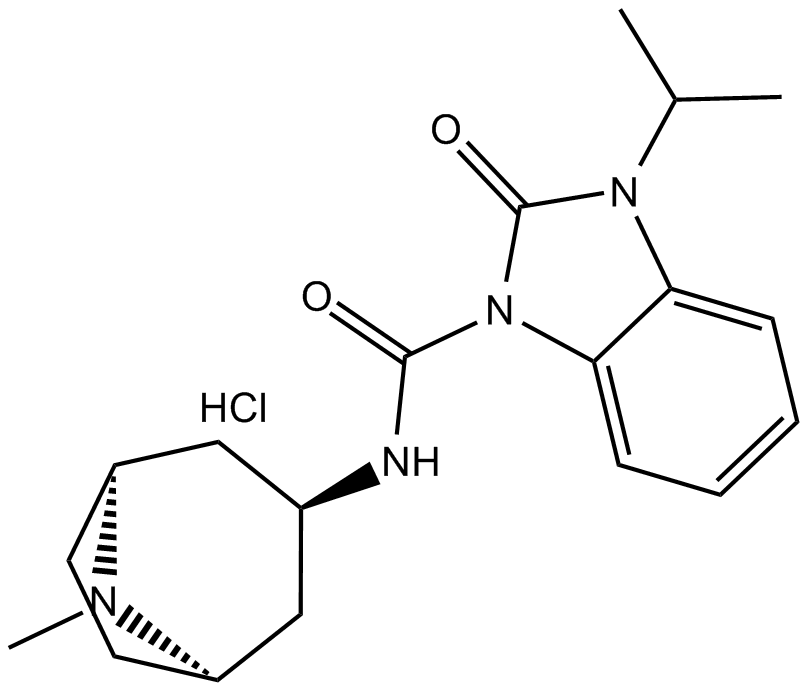
BIMU 8
BIMU 8 is an agonist of 5-HT4 with Ki values of 33.9 ± 8.0 nM and 12.6 ± 0.9 nM in guinea pig ileum and striatum, respectively [1, 2].
As a member of the seven transmembrane spanning G-protein-coupled family of receptors, the 5-HT4 receptor is positively coupled to adenylate cyclase. It exists in two isoforms (5-HT4S and 5-HT4L). These two isoforms differ in the sequence and length of their carboxy termini [3].
BIMU 8 significantly decreased the K+ current in colliculi neurons. This suggested a 5-HT4 receptor-mediated effect [4]. In neurons, BIMU 8 at concentrations ranging from 0.003-0.1 µM increased EPSP amplitude but did not change membrane potential. The EPSP potentiation induced by BIMU 8 was blocked by tropisetron (1 µM), a 5-HT3/5-HT4 receptor antagonist. But ondansetron (1 µM), a 5-HT3 receptor antagonist did not blocked the EPSP potentiation induced by BIMU 8 [5].
In the hot-plate test, BIMU 8 injected i.p. in the range of doses of 20-30 mg/kg significantly induced an increase in the pain threshold. 15 min after administration, the antinociceptive effect reached a maximum and hence diminished. This effect disappeared within 45 min. Choline uptake blocker HC-3 (1 µg per mouse i.c.v.), antimuscarinic drug atropine (5 mg/kg i.p.), 5-HT4 antagonists SDZ 205-557 (10 mg/kg i.p.) and GR 125487 (20 mg/kg i.p.) completely prevented the antinociception of BIMU 8 [1].
References:
[1]. Ghelardini C, Galeotti N, Casamenti F, et al. Central cholinergic antinociception induced by 5HT4 agonists: BIMU 1 and BIMU 8. Life sciences, 1996, 58(25): 2297-2309.
[2]. Yoshikawa T, Yoshida N, Mine Y, et al. Affinity of mosapride citrate, a new gastroprokinetic agent, for 5-HT4 receptors in guinea pig ileum. The Japanese Journal of Pharmacology, 1998, 77(1): 53-59.
[3]. Hegde SS, Eglen RM. Peripheral 5-HT4 receptors. The FASEB journal, 1996, 10(12): 1398-1407.
[4]. Fagni L, Dumuis A, Sebben M, et al. The 5-HT4 receptor subtype inhibits K+ current in colliculi neurones via activation of a cyclic AMP-dependent protein kinase. British journal of pharmacology, 1992, 105(4): 973-979.
[5]. Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. American Journal of Physiology-Gastrointestinal and Liver Physiology, 1994, 266(2): G230-G238.
| Physical Appearance | Off-white solid |
| Storage | Store at -20°C |
| M.Wt | 378.9 |
| Cas No. | 134296-40-5 |
| Formula | C19H26N4O2·HCl |
| Solubility | <37.89mg/ml in DMSO; <28.42mg/ml in H2O |
| Chemical Name | 3-isopropyl-N-((1R,3r,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-1-carboxamide hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | CC(C)N(c(cccc1)c1N1C(N[C@@H]2C[C@H](CC3)N(C)[C@H]3C2)=O)C1=O.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure