Risedronate Sodium
Risedronate Sodium (CAS No. 115436-72-1) is a bisphosphonate drug, with its core target being farnesyl pyrophosphate synthase (FPPS), a key enzyme in the mevalonate pathway. By inhibiting this enzyme, it blocks the synthesis of isoprenoid lipids in osteoclasts, thereby suppressing bone resorption. Meanwhile, it can regulate the WNT/β-catenin signaling pathway and target alveolar macrophages (the drug is repurposed for the treatment of emphysema), and synergistically modulates bone metabolism with vitamin D₃. Its activity is characterized by functional effects such as bone resorption inhibition, bone mineral density enhancement, and apoptosis induction.
Common application concentrations are as follows: 0.1–1000 μg/mL for in vitro cell experiments (Calu-3 cell cytotoxicity and uptake assays); the loading concentration of nano-delivery systems/microsphere formulations corresponds to an encapsulation efficiency of 86.12%–92.4%. For animal experiments, the dosages are 0.1 mg/kg/day via oral administration (osteoporosis model), 100–200 mg/kg via inhalation (rat osteoporosis model), and 500 μg/kg/day via intratracheal administration (emphysema model).
The clinically effective therapeutic concentrations correspond to multiple administration routes: for glucocorticoid-induced osteoporosis (GIO) and rheumatoid arthritis complicated with GIO, the oral dosage is 75 mg per month, or a daily dose combined with vitamin D₃. For osteoporosis treatment, the inhaled formulation is administered at 0.1 mg/kg/day (RIS) plus 45 IU/kg/day (VITD₃). For emphysema treatment, the reference dosage is 500 μg/kg/day via intratracheal administration.
Its biological activities are manifested as a significant increase in lumbar spine bone mineral density (a 3.49% increase compared with the placebo group), reduction of bone turnover markers (TRACP-5b and BAP), and induction of alveolar macrophage apoptosis to alleviate emphysema. The oral bioavailability is less than 1%, while inhalation or nano-formulations can improve bioavailability and reduce gastrointestinal adverse reactions. It has a favorable safety profile and is indicated for the treatment of osteoporosis and emphysema.
References:
[1] Fujieda Y, Horita T, Nishimoto N, Tanimura K, Amasaki Y, Kasahara H, Furukawa S, Takeda T, Fukaya S, Matsui K, Tsutsumi A, Furusaki A, Sagawa A, Katayama K, Takeuchi K, Katsumata K, Kurita T, Shane P, Kato M, Oku K, Yasuda S, Takahata M, Iwasaki N, Atsumi T. Efficacy and safety of sodium RISedronate for glucocorticoid-induced OsTeoporosis with rheumaTOid arthritis (RISOTTO study): A multicentre, double-blind, randomized, placebo-controlled trial. Mod Rheumatol. 2021 May;31(3):593-599. doi: 10.1080/14397595.2020.1812835. Epub 2020 Oct 2. PMID: 32820698.
[2] Elkady OA, Saleh LM, Tadros MI, El-Laithy HM. Nebulization of Risedronate Sodium Microspheres for Potential Attenuation of Pulmonary Emphysema: a Promising New Insight of Alveolar Macrophage Apoptosis. AAPS PharmSciTech. 2021 Jul 7;22(5):202. doi: 10.1208/s12249-021-02078-8. PMID: 34235597.
[3] Elsayyad NME, Gomaa I, Salem MA, Amer R, El-Laithy HM. Efficient lung-targeted delivery of risedronate sodium/vitamin D3 conjugated PAMAM-G5 dendrimers for managing osteoporosis: Pharmacodynamics, molecular pathways and metabolomics considerations. Life Sci. 2022 Nov 15;309:121001. doi: 10.1016/j.lfs.2022.121001. Epub 2022 Sep 27. PMID: 36174709.
[4] Sultana N, Ali A, Waheed A, Jabi B, Yaqub Khan M, Mujeeb M, Sultana Y, Aqil M. Dissolving microneedle transdermal patch loaded with Risedronate sodium and Ursolic acid bipartite nanotransfersomes to combat osteoporosis: Optimization, characterization, in vitro and ex vivo assessment. Int J Pharm. 2023 Sep 25;644:123335. doi: 10.1016/j.ijpharm.2023.123335. Epub 2023 Aug 18. PMID: 37597597.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 305.09 |
| Cas No. | 115436-72-1 |
| Formula | C7H10NNaO7P2 |
| Solubility | insoluble in EtOH; insoluble in DMSO; ≥10.17 mg/mL in H2O with gentle warming |
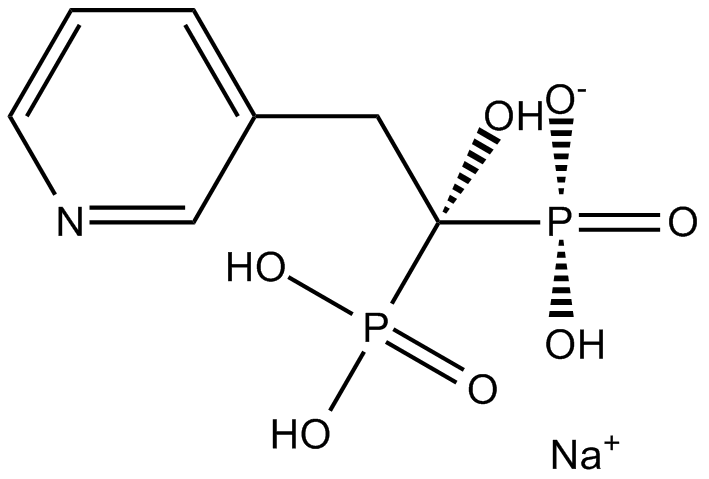
| Chemical Name | sodium;hydroxy-(1-hydroxy-1-phosphono-2-pyridin-3-ylethyl)phosphinate |
| SDF | Download SDF |
| Canonical SMILES | [O-]P(C(Cc1cnccc1)(O)P(O)(O)=O)(O)=O.[Na+] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure