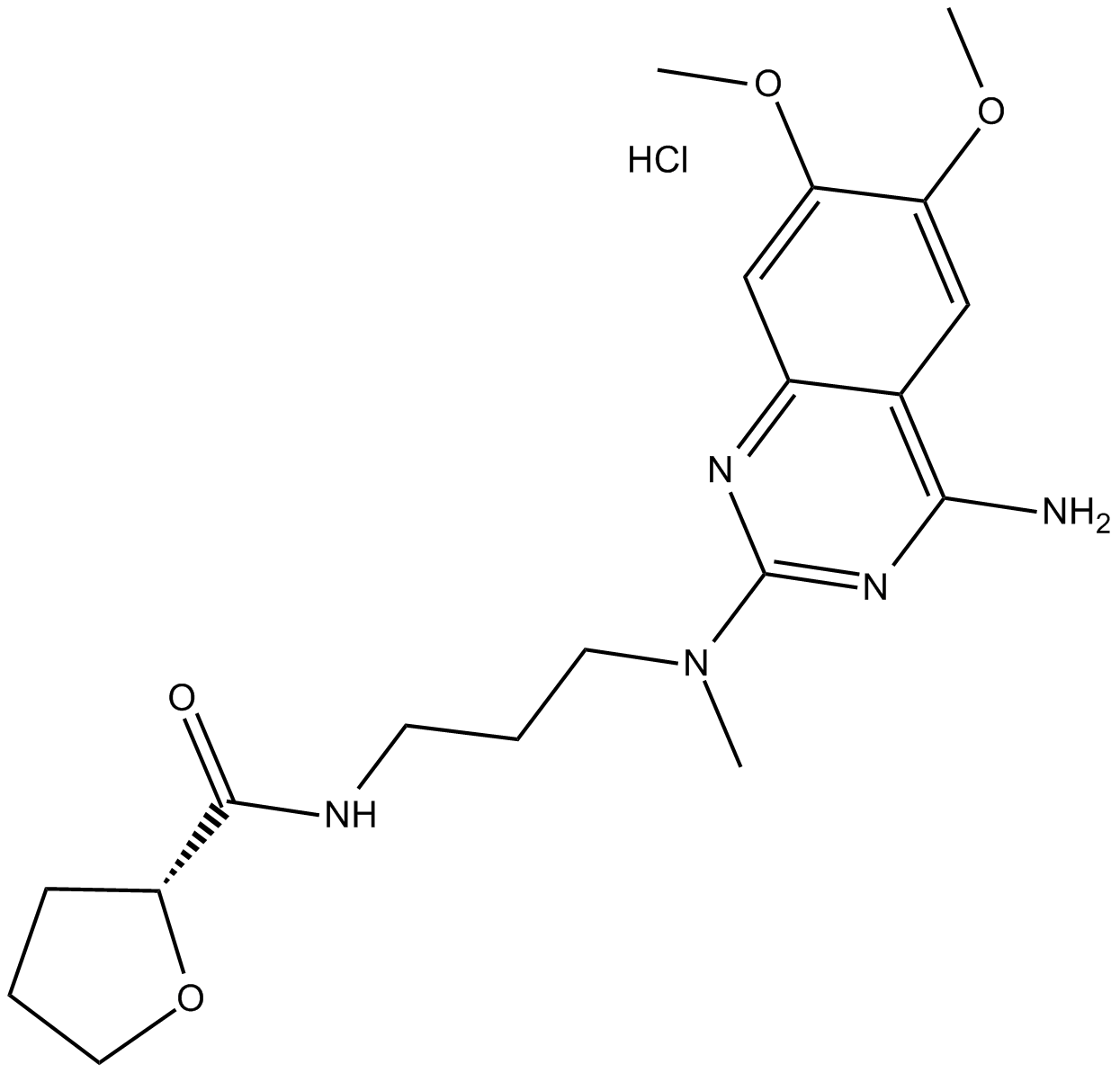
Alfuzosin HCl
Alfuzosin hydrochloride (CAS No. 81403-68-1) is a second-generation selective α?-adrenergic receptor antagonist. Its core biological activity is to relax the smooth muscle of the prostate, bladder neck, and urethra to improve lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH). Its targets include the α?A, α?B, and α?D receptor subtypes (with the α?A receptor in prostatic tissue as the main site of action); application concentrations: for in vitro spectroscopic analysis (fluorometric / spectrophotometric), the linear ranges are 1.0–16.0 ng/mL and 1–15 μg/mL, respectively. In formulation studies, in vitro release tests use 0.1 N HCl as the medium, with a drug loading of 10 mg per dosage unit; effective therapeutic concentrations correspond to clinical doses: immediate-release (IR) formulations 2.5 mg 2–3 times daily, extended-release (ER) formulations 5 mg twice daily or 10 mg once daily, with no need for dose titration. The clinical peak plasma concentration is 2.0–12.0 ng/mL, urinary concentration 1.0–5.0 ng/mL, oral bioavailability 64%, unaffected by food, predominantly hepatic metabolism, protein binding rate 90%, half-life about 5 hours, with good safety and a lower incidence of cardiovascular adverse effects than other second-generation α? receptor antagonists.
References:
[1] Lee M. Alfuzosin hydrochloride for the treatment of benign prostatic hyperplasia. Am J Health Syst Pharm. 2003 Jul 15;60(14):1426-39. doi: 10.1093/ajhp/60.14.1426. Erratum in: Am J Health Syst Pharm. 2004 Mar 1;61(5):437. PMID: 12892027.
[2] Abd El-Aziz MF, Ismail S, Tadros MI, Elnabarawi MA. Alfuzosin hydrochloride-loaded low-density gastroretentive sponges: development, in vitro characterization and gastroretentive monitoring in healthy volunteers via MRI. Pharm Dev Technol. 2020 Jun;25(5):566-578. doi: 10.1080/10837450.2020.1720235. Epub 2020 Feb 6. PMID: 31967910.
[3] Elama HS, Shalan SM, El-Shabrawy Y, Eid MI, Zeid AM. Utilization of a micellar matrix for simultaneous spectrofluorimetric estimation of alfuzosin hydrochloride and vardenafil hydrochloride. Spectrochim Acta A Mol Biomol Spectrosc. 2022 Feb 5;266:120420. doi: 10.1016/j.saa.2021.120420. Epub 2021 Sep 27. PMID: 34619505.
[4] Alqahtani A, Alqahtani T, Ramzy S. Utilization of absorbance subtraction and ratio difference green spectrophotometric methods for the quantification of alfuzosin hydrochloride and tadalafil in their binary mixture. BMC Chem. 2024 May 9;18(1):96. doi: 10.1186/s13065-024-01201-7. PMID: 38725069; PMCID: PMC11080132.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 425.91 |
| Cas No. | 81403-68-1 |
| Formula | C19H27N5O4·HCl |
| Solubility | ≥19 mg/mL in DMSO; ≥3 mg/mL in EtOH with ultrasonic; ≥47.8 mg/mL in H2O |
| Chemical Name | N-[3-[(4-amino-6,7-dimethoxyquinazolin-2-yl)-methylamino]propyl]oxolane-2-carboxamide;hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | CN(CCCNC(C1OCCC1)=O)c1nc(cc(c(OC)c2)OC)c2c(N)n1.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure