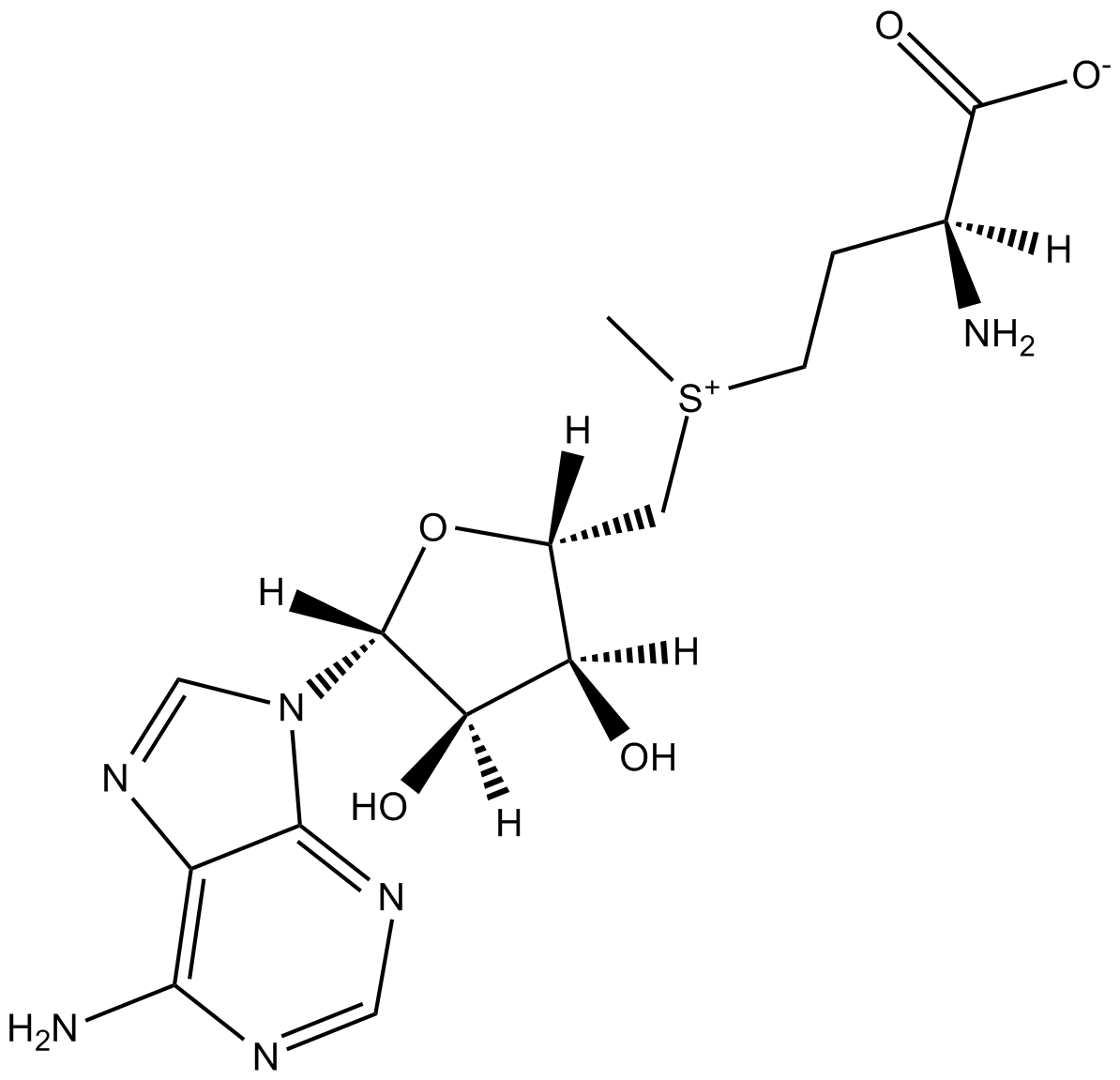
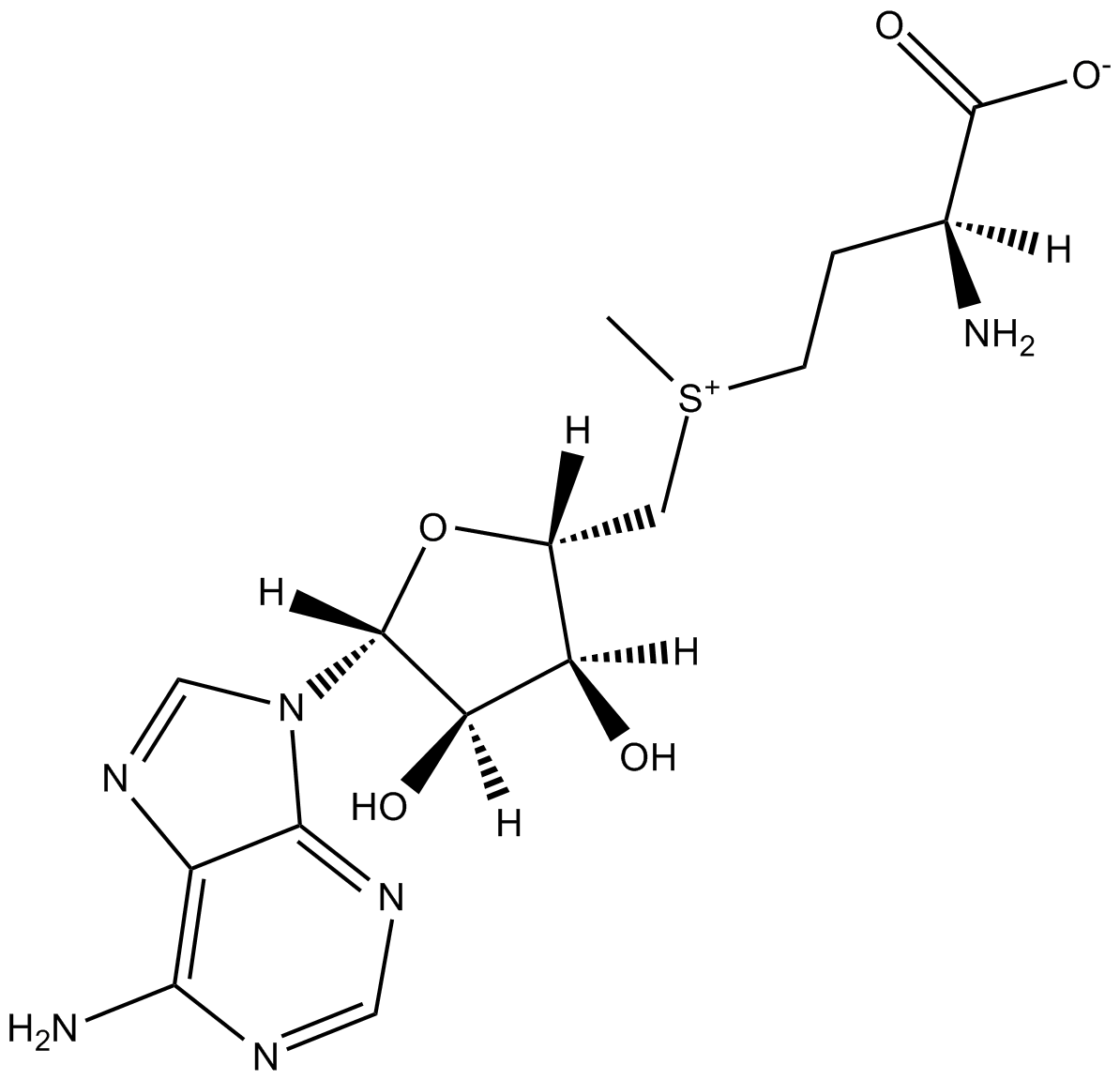
S-Adenosylmethionine (SAM)
S-Adenosylmethionine (SAMe, CAS No. 29908-03-0) is a key endogenous metabolite. Its core targets include various methyltransferases (such as DNA methyltransferases DNMTs, histone methyltransferases EZH2/G9a, and RNA methyltransferases METTL3/METTL14), cystathionine β-synthase (CBS), methionine synthase (MS), and SAMTOR (a sensor of the mTORC1 pathway). It participates in the methylation of DNA, RNA, proteins, and phospholipids by donating methyl groups, and simultaneously regulates the transsulfuration pathway and cell growth signals.
The affinity of relevant methyltransferases for SAMe is characterized by the KM value, with a range of 0.06 μM to 240 μM (e.g., the KM value of METTL3/METTL14 is approximately 100 nM, and that of DNMT3a is 0.2–2.56 μM). The commonly used concentration in experiments is 1–100 μM (for cell methylation regulation assays and metabolic pathway studies), and approximately 7 μM is typically used in SAMTOR binding experiments (corresponding to the dissociation constant).
The clinically effective therapeutic concentration corresponds to the following administration regimens: oral enteric-coated tablets at a daily dose of 200–1600 mg (administered in 1–2 divided doses) for the treatment of depression, osteoarthritis, and liver diseases; or intravenous/intramuscular injection at a daily dose of 200–800 mg for severe cases. The plasma peak concentration is reached 3–6 hours after oral administration, and SAMe can cross the blood-brain barrier to increase its concentration in cerebrospinal fluid.
Its biological activities are reflected in regulating epigenetics and transcriptome, improving neurotransmitter metabolism (exerting antidepressant effects), promoting cartilage repair (exerting anti-osteoarthritis effects), enhancing hepatic glutathione synthesis (exerting hepatoprotective effects), and maintaining cellular SAM homeostasis to balance cell growth and survival. It has a favorable safety profile, with only mild adverse reactions such as gastrointestinal discomfort reported.
References:
[1] Bottiglieri T, Hyland K, Reynolds EH. The clinical potential of ademetionine (S-adenosylmethionine) in neurological disorders. Drugs. 1994 Aug;48(2):137-52. doi: 10.2165/00003495-199448020-00002. PMID: 7527320.
[2] Chavez M. SAMe: S-Adenosylmethionine. Am J Health Syst Pharm. 2000 Jan 15;57(2):119-23. doi: 10.1093/ajhp/57.2.119. PMID: 10688238.
[3] Xing Z, Tu BP. Mechanisms and rationales of SAM homeostasis. Trends Biochem Sci. 2025 Mar;50(3):242-254. doi: 10.1016/j.tibs.2024.12.009. Epub 2025 Jan 15. PMID: 39818457; PMCID: PMC11890959.
| Storage | Store at -20°C |
| M.Wt | 398.44 |
| Cas No. | 29908-03-0 |
| Formula | C15H22N6O5S |
| Synonyms | S-Adenosyl-L-methionine;Ademetionine; SAMe; SAM-e; AdoMet |
| Solubility | insoluble in EtOH; ≥108 mg/mL in H2O; ≥110.8 mg/mL in DMSO |
| Chemical Name | (2S)-2-amino-4-((((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(methyl)sulfonio)butanoate |
| SDF | Download SDF |
| Canonical SMILES | O[C@H]1[C@H](N2C=3C(N=C2)=C(N)N=CN3)O[C@H](C[S+](CC[C@@H](C([O-])=O)N)C)[C@H]1O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Animal experiment:[1] | |
|
Animal models |
Mice and rats |
|
Dosage form |
12.5, 25, 50, 100 and 200 mg/kg Subcutaneous injection |
|
Applications |
Ademetionine dose-dependently decreased immobility time in the forced swimming test in mice and rats, and the effect could be antagonized by haloperiodol. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Bottiglieri T. Ademetionine (S-adenosylmethionine) neuropharmacology: implications for drug therapies in psychiatric and neurological disorders. Expert Opinion on Investigational Drugs, 1997, 6(4): 417-426. |
|
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure