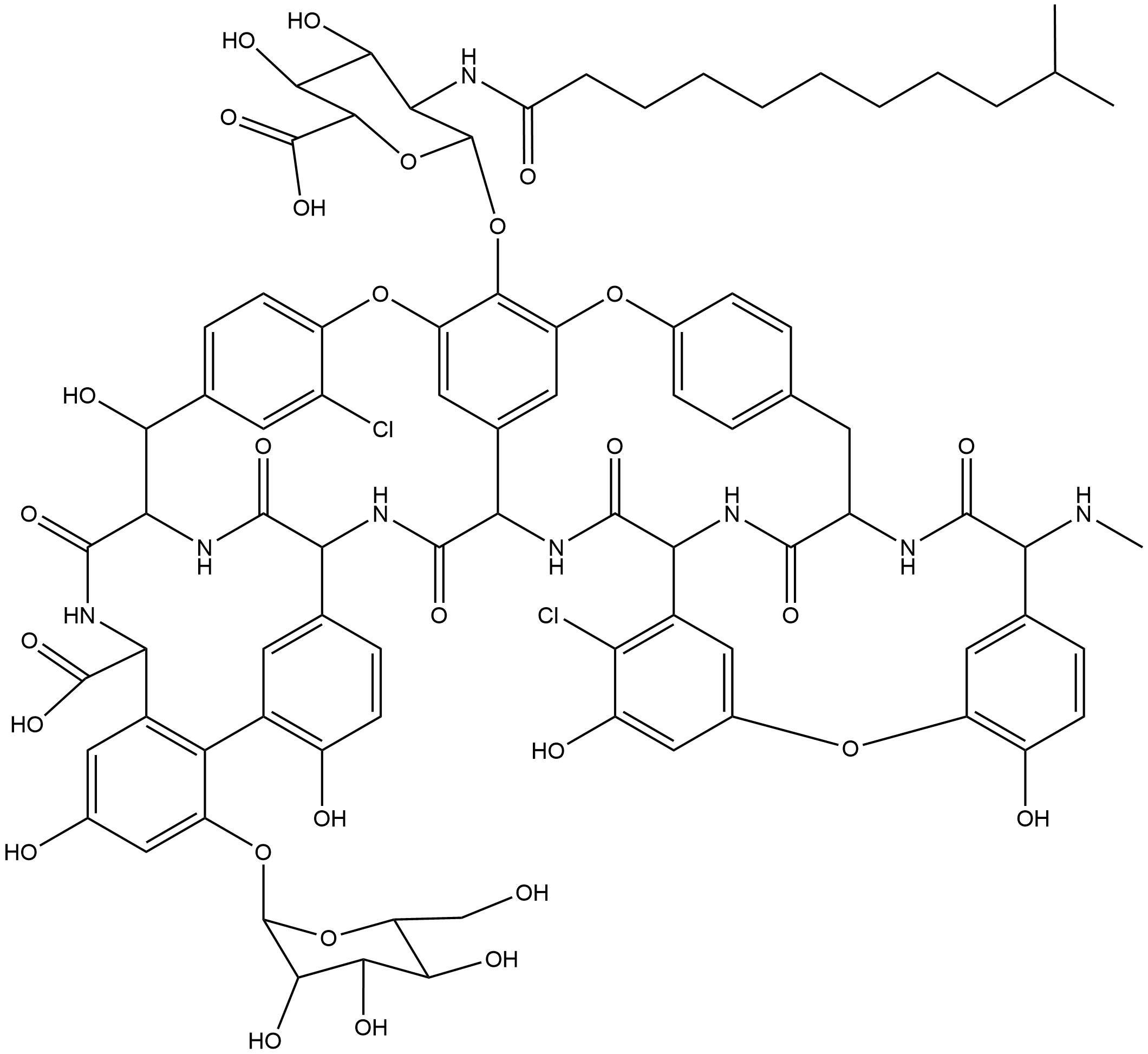
A40926
A40926 (CAS No. 102961-72-8) is the natural precursor of the glycopeptide bactericidal antibiotic dalbavancin (CAS No. 171500-79-1, Cat. No.: BA1035). Its core bioactivity is to inhibit the growth of Gram-positive bacteria and Neisseria gonorrhoeae, and its target is the bacterial cell wall synthesis pathway (binding to the D-alanyl-D-alanine structure of peptidoglycan precursors and blocking peptidoglycan cross-linking). Its biosynthesis is positively regulated by the regulatory genes dbv3 (LuxR-like) and dbv4 (StrR-like). The MIC is pathogen-specific: for Staphylococcus aureus, MIC?? is 0.25~0.5 μg/mL; for Streptococcus pyogenes, 0.06 μg/mL; for Enterococcus faecium, 0.06~0.25 μg/mL; for clinical isolates of Neisseria gonorrhoeae, MIC??/MIC?? are 1 μg/mL/2 μg/mL, respectively (significantly better than vancomycin (CAS No. 1404-90-6, Cat. No.: C6417) and teicoplanin (CAS No. 61036-62-2, Cat. No.: B1847)); for coagulase-negative staphylococci, the MIC range is 0.06~8 μg/mL. Common application concentrations: for in vitro antibacterial assays, 0.004~64 μg/mL; in animal experiments, the ED?? in a mouse septicemia model by subcutaneous injection is 0.33~1.9 mg/kg. In fermentation production, engineered strains can reach yields of 332 mg/L~800 mg/L in optimized media (containing glucose, maltodextrin, soybean meal, etc.). Its semi-synthetic derivative dalbavancin is used clinically for Gram-positive bacterial infections. Based on the in vitro antibacterial activity and animal experiment data of A40926, it is inferred that the clinically effective therapeutic concentration corresponds to a fermentation broth concentration of 300~800 mg/L in fermentation production. It remains effective against multidrug-resistant Neisseria gonorrhoeae, MRSA, etc., and N-acylaminoglucuronic acid glycoside derivatives show better activity against coagulase-negative staphylococci than the parent compound.
References:
[1] Goldstein BP, Selva E, Gastaldo L, Berti M, Pallanza R, Ripamonti F, Ferrari P, Denaro M, Arioli V, Cassani G. A40926, a new glycopeptide antibiotic with anti-Neisseria activity. Antimicrob Agents Chemother. 1987 Dec;31(12):1961-6. doi: 10.1128/AAC.31.12.1961. PMID: 2964225; PMCID: PMC175835.
[2] Selva E, Goldstein BP, Ferrari P, Pallanza R, Riva E, Berti M, Borghi A, Beretta G, Scotti R, Romanò G, et al. A40926 aglycone and pseudoaglycones: preparation and biological activity. J Antibiot (Tokyo). 1988 Sep;41(9):1243-52. doi: 10.7164/antibiotics.41.1243. PMID: 3053550.
[3] Sosio M, Donadio S. Understanding and manipulating glycopeptide pathways: the example of the dalbavancin precursor A40926. J Ind Microbiol Biotechnol. 2006 Jul;33(7):569-76. doi: 10.1007/s10295-006-0124-1. Epub 2006 Apr 26. PMID: 16761167.
[4] Yushchuk O, Andreo-Vidal A, Marcone GL, Bibb M, Marinelli F, Binda E. New Molecular Tools for Regulation and Improvement of A40926 Glycopeptide Antibiotic Production in Nonomuraea gerenzanensis ATCC 39727. Front Microbiol. 2020 Jan 21;11:8. doi: 10.3389/fmicb.2020.00008. PMID: 32038594; PMCID: PMC6985074.
[5] Yan B, Gao W, Tian L, Wang S, Dong H. Production enhancement of the glycopeptide antibiotic A40926 by an engineered Nonomuraea gerenzanensis strain. Biotechnol Lett. 2022 Feb;44(2):259-269. doi: 10.1007/s10529-021-03210-1. Epub 2021 Nov 26. PMID: 34826003.
[6] Andreo-Vidal A, Yushchuk O, Marinelli F, Binda E. Cross-Talking of Pathway-Specific Regulators in Glycopeptide Antibiotics (Teicoplanin and A40926) Production. Antibiotics (Basel). 2023 Mar 24;12(4):641. doi: 10.3390/antibiotics12040641. PMID: 37107003; PMCID: PMC10135024.
[7] Zhukrovska K, Binda E, Fedorenko V, Marinelli F, Yushchuk O. The Impact of Heterologous Regulatory Genes from Lipodepsipeptide Biosynthetic Gene Clusters on the Production of Teicoplanin and A40926. Antibiotics (Basel). 2024 Jan 24;13(2):115. doi: 10.3390/antibiotics13020115. PMID: 38391501; PMCID: PMC10886168.
| Physical Appearance | A solid |
| Storage | -20°C |
| M.Wt | 1732.53 |
| Cas No. | 102961-72-8 |
| Formula | C83H88Cl2N8O29 |
| Synonyms | Dalbavancin Impurity; Dalbavancin Nucleus HCl(Main A40926) |
| Chemical Name | 56-(((2S,3R,4R,5S,6S)-6-carboxy-4,5-dihydroxy-3-(10-methylundecanamido)tetrahydro-2H-pyran-2-yl)oxy)-5,55-dichloro-6,11,34,40,44-pentahydroxy-15-(methylamino)-2,16,36,50,51,59-hexaoxo-42-(((2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-p |
| SDF | Download SDF |
| Canonical SMILES | CNC1c2ccc(O)c(Oc3cc(O)c(Cl)c(c3)C3NC(=O)C(Cc4ccc(Oc5cc6cc(Oc7ccc(cc7Cl)C(O)C7NC(=O)C(NC(=O)C6NC3=O)c3ccc(O)c(c3)-c3c(O[C@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]6O)cccc3C(NC7=O)C(O)=O)c5O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3NC(=O)CCCCCCCCC(C)C)C(O)=O)cc4)NC1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |