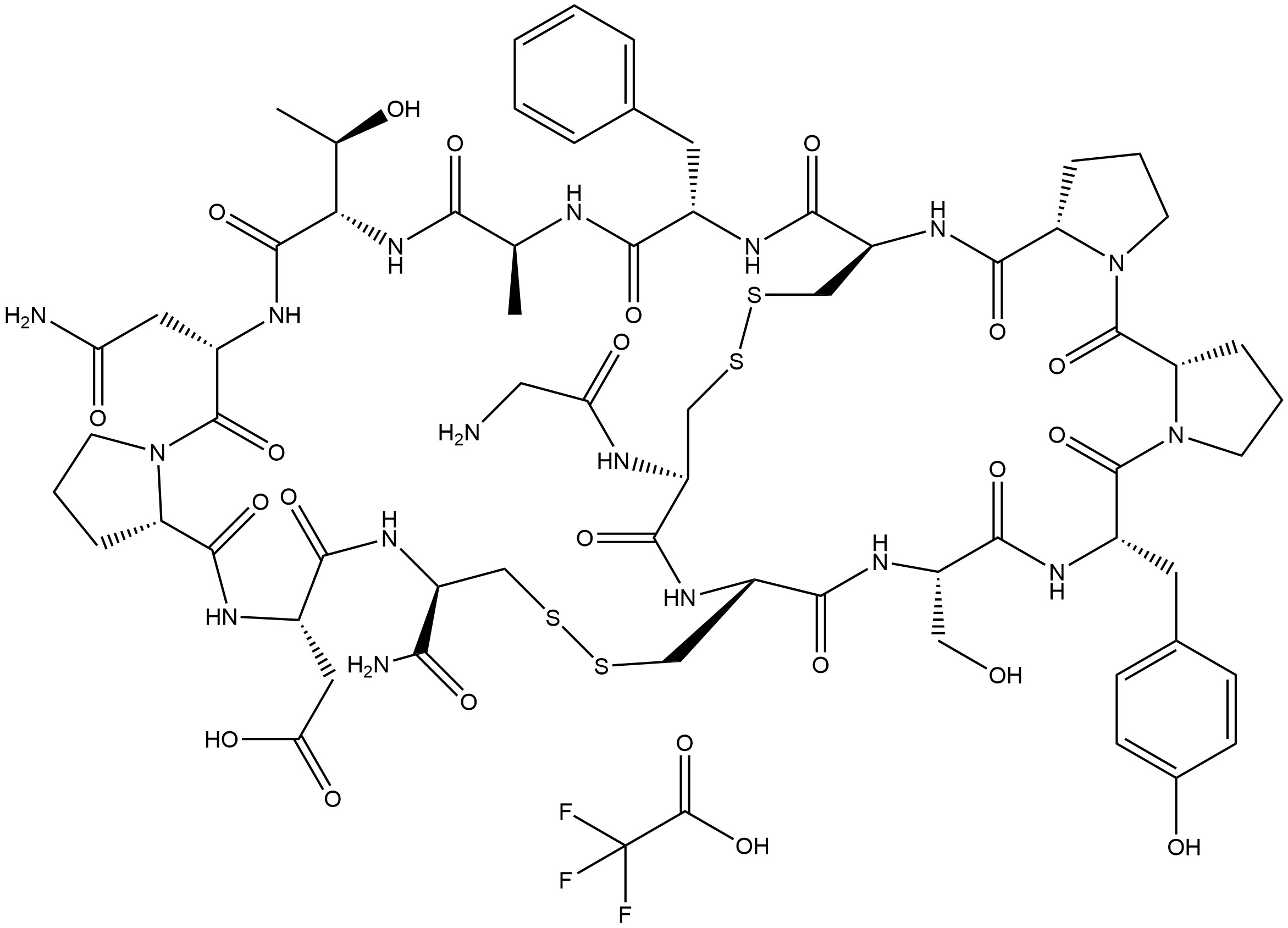
α-Conotoxin AuIB TFA
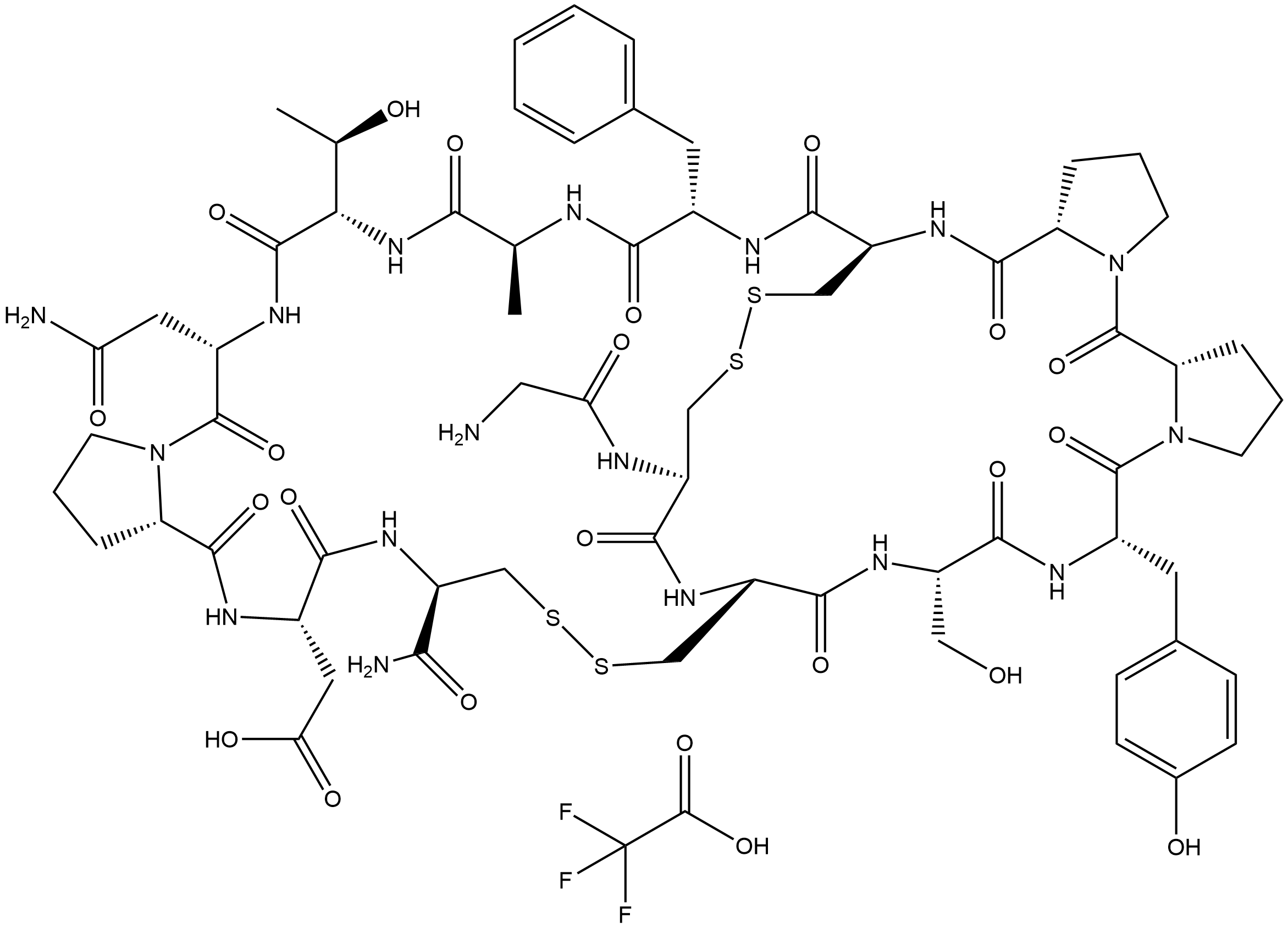
α-Conotoxin AuIB TFA is the trifluoroacetic acid salt of α-Conotoxin AuIB(CAS 216299-21-7, catalog NO.B5376), a peptide that selectively and non-competitively antagonizes the α3β4 subtype of nicotinic acetylcholine receptors (nAChR) at micromolar potency, and also targets the GABAB receptor, leading to downstream inhibition of N-type voltage-gated calcium channels (CaV2.2). It exerts specific non-competitive antagonism on α3β4 nAChR with micromolar potency and is currently the only known α-conotoxin that exclusively inhibits this subtype. The core mechanism involves the Phe-9 residue of AuIB, which forms a π-π stacking interaction with Trp-59 in the Loop D region of the β4 subunit and a cation-π interaction with β4-Lys-61; these two residues together constitute a specific binding pocket. Meanwhile, Phe-9 can promote the formation of disulfide contacts between Cys2/8 of AuIB and Cys192/193 of the α3 subunit to stabilize the toxin-receptor complex, and the loss of such contacts weakens inhibitory efficacy. Pro-6 is an essential residue for AuIB to form a 3₁₀ helix turn and maintain its globular three-dimensional structure (the [P6A] mutation disrupts the secondary structure and leads to complete loss of inhibitory activity), while Gly-1 forms a salt bridge between the N-terminal NH₃⁺ and β4-Asp-172 (the [G1A] mutation causes a moderate decrease in inhibitory activity). Additionally, the ribbon isomer of AuIB (with I-IV, II-III disulfide bond connectivity) exhibits stronger inhibitory potency on α3β4 nAChR, converts to competitive antagonism, and shows stoichiometry-dependent blockade, unlike the native globular isomer (with I-III, II-IV connectivity). The [F9A] mutant loses key interactions, resulting in over a 10-fold decrease in inhibitory activity and altered subtype selectivity (exerting weak inhibition on α9α10 nAChR and slight potentiation on α7 nAChR). For its analgesic activity, AuIB indirectly inhibits CaV2.2 by activating the GABAB receptor; this pathway has stronger analgesic potency than its nAChR inhibitory activity and demonstrates efficacy in animal pain models. The binding pocket composed of Trp-59 (highly conserved) and Lys-61 (β4 subtype-specific residue) in the Loop D of the β4 subunit is critical for AuIB to selectively recognize α3β4 nAChR (residues at the corresponding positions in other nAChR subtypes, such as β2, are Thr, which cannot form effective cation-π interactions, thus not being inhibited by AuIB). This provides a core target for structural optimization to retain analgesic activity while reducing nAChR-related side effects.
References:
[1] Grishin AA, Cuny H, Hung A, Clark RJ, Brust A, Akondi K, Alewood PF, Craik DJ, Adams DJ. Identifying key amino acid residues that affect α-conotoxin AuIB inhibition of α3β4 nicotinic acetylcholine receptors. J Biol Chem. 2013 Nov 29;288(48):34428-42. doi: 10.1074/jbc.M113.512582. Epub 2013 Oct 7. PMID: 24100032; PMCID: PMC3843058.
| Storage | Desiccate at -20°C |
| M.Wt | 1686.79 |
| Cas No. | 216299-21-7 (free base) |
| Formula | C65H89N17O21S4 • CF3COOH |
| Chemical Name | 2,2,2-trifluoroacetic acid--2-((5aS,11S,14S,17R,22R,25S,27aS,33S,36S,39S,42S,45R,47aS,52R)-33-(2-amino-2-oxoethyl)-52-(2-aminoacetamido)-42-benzyl-22-carbamoyl-11-(4-hydroxybenzyl)-36-((R)-1-hydroxyethyl)-14-(hydroxymethyl)-39-methyl-5,10,13,16,24,27,32,3 |
| SDF | Download SDF |
| Canonical SMILES | O=C1[C@@]2([H])N(CCC2)C([C@@H](NC([C@@H](NC([C@@]3([H])NC([C@H](CSSC[C@@H](C(N[C@H](C(N[C@H](C(N[C@@](C(N[C@H](C(N4[C@](CCC4)([H])C(N[C@H](C(N[C@@H](CSSC3)C(N)=O)=O)CC(O)=O)=O)=O)CC(N)=O)=O)([H])[C@H](O)C)=O)C)=O)CC5=CC=CC=C5)=O)NC([C@@]6([H])N1CCC6)=O)NC |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure