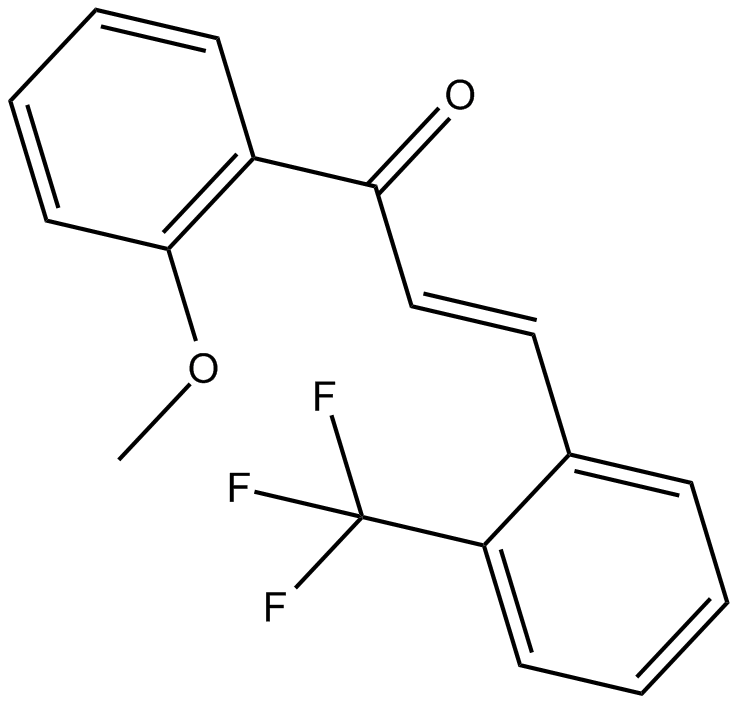
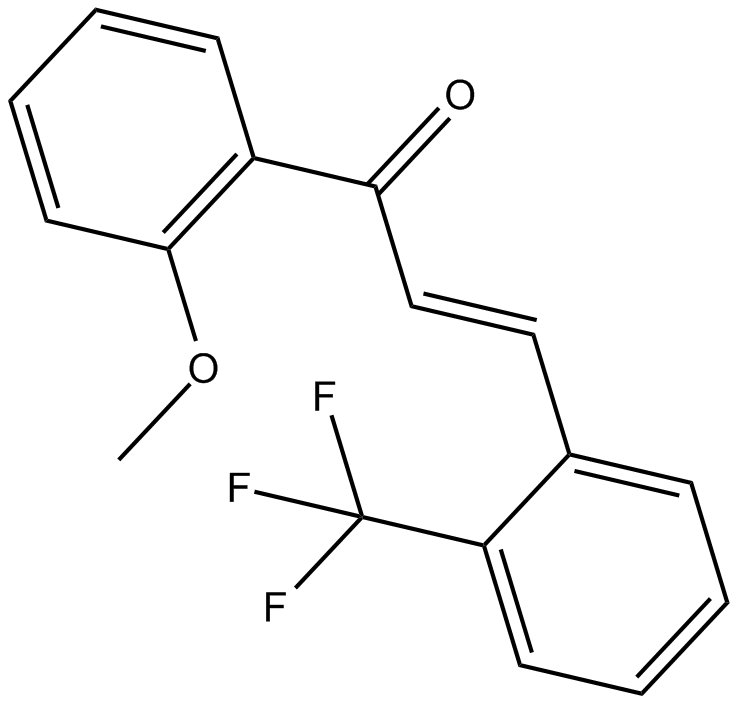
2-Trifluoromethyl-2'-methoxychalcone
2-Trifluoromethyl-2'-methoxychalcone is a Nrf2 activator.
Nrf2-mediated activation of antioxidant response element is a key process of molecular mechanisms regulating the protective function of phase II detoxification and antioxidant enzymes against oxidative stress, carcinogenesis, and inflammation.
In vitro: The expression of luciferase gene under the control of NQO1-ARE sequence was measured using stably transfected Beas-2B cells treated with 2-trifluoromethyl-2'-methoxychalcone. Results showed that the exposure to 2-trifluoromethyl-2'-methoxychalcone led to a significant concentration-dependent increase in luciferase activity. Moreover, Beas-2B cells were coincubated with 2-trifluoromethyl-2'-methoxychalcone and with or without N-acetylcysteine, and it was found that 2-trifluoromethyl-2'-methoxychalcone could potentially increase the expression of Nr2-regulated antioxidant genes in the presence of N-acetylcysteine [1].
In vivo: C57BL/6 mice were treated with a single dose of vehicle or 2-trifluoromethyl-2'-methoxychalcone or sulforaphane as the positive control, and small intestines were harvested 24 h later. Results showed that the expression of GCLM and NQO1 in the small intestine of mice treated with 2-trifluoromethyl-2'-methoxychalcone was 6-fold and 10-fold higher compared to vehicle, respectively. In addition, the expression of GCLM and NQO1 in the small intestine treated with 2-trifluoromethyl-2'-methoxychalcone was 3-fold and 5-fold higher compared to sulforaphane, respectively [1].
Clinical trial: Up to now, 2-trifluoromethyl-2'-methoxychalcone is still in the preclinical development stage.
Reference:
[1] V. Kumar, S. Kumar, M. Hassan, et al. Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. Journal of Medicinal Chemistry 54(12), 4147-4159 (2011).
| Physical Appearance | A solution in acetate. To change the solvent, simply evaporate the acetate containing under a gentle stream of nitrogen and immediately add the solvent of choice. |
| Storage | Store at -20°C |
| M.Wt | 306.3 |
| Cas No. | 1309371-03-6 |
| Formula | C17H13F3O2 |
| Solubility | ≤11mg/ml in ethanol;5mg/ml in DMSO;14mg/ml in dimethyl formamide |
| Chemical Name | 1-(2-methoxyphenyl)-3-[2-(trifluoromethyl)phenyl]-2E-propen-1-one |
| SDF | Download SDF |
| Canonical SMILES | COc(cccc1)c1C(/C=C/c1c(C(F)(F)F)cccc1)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure