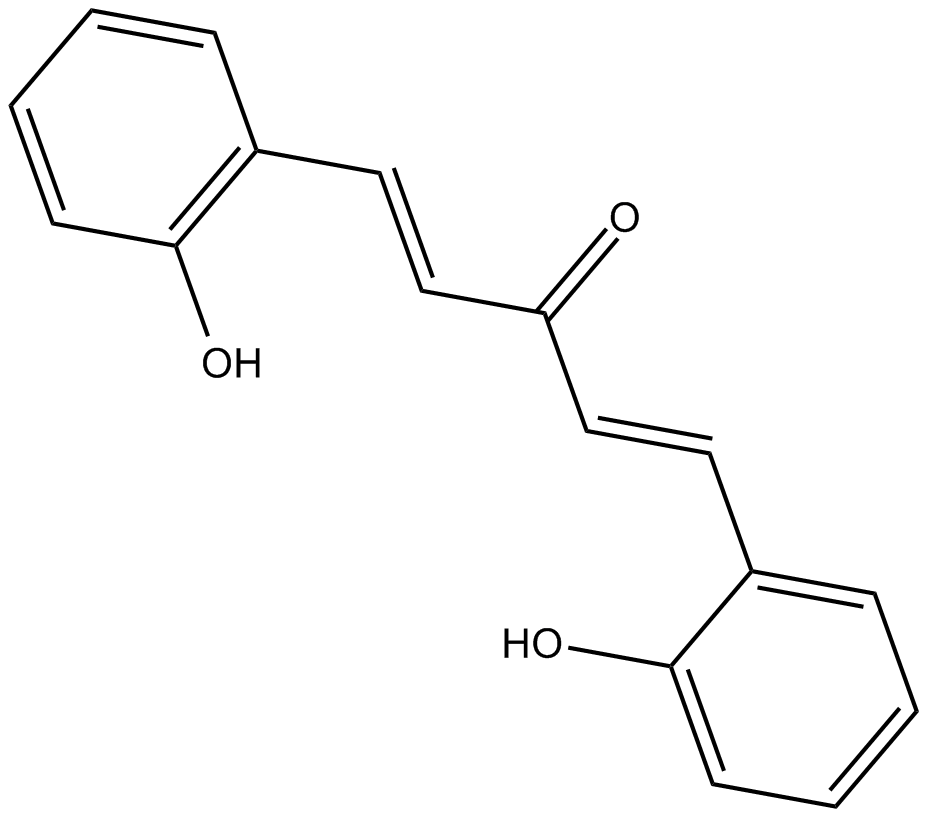
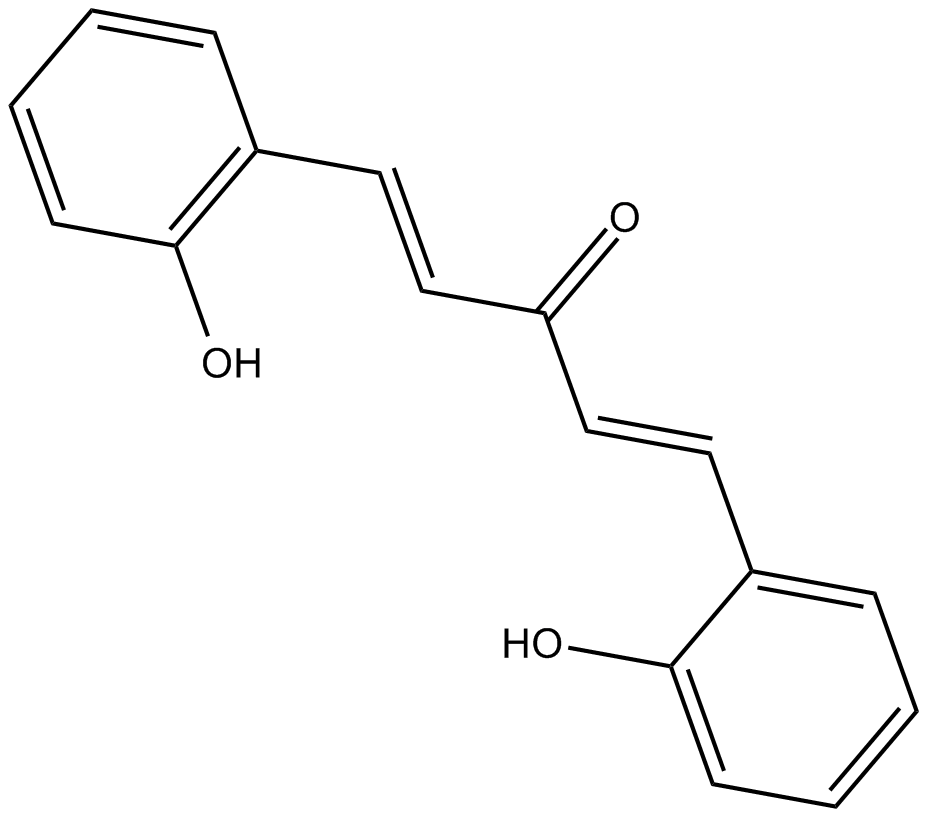
2-HBA
2-HBA is an inducer of the Keap1-Nrf2-ARE pathway.
Keap1-Nrf2-ARE directly react with Keap1, the sensor protein for inducers, leads to enhanced transcription of phase 2 genes and protection against oxidant and electrophile toxicities.
In vitro: 2-HBA could markedly increase the activities of NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1) and glutathione reductase, the levels of total glutathione, as well as the phase 2 response markers. In addition, at high concentrations 2-HBA caused G2/M cell cycle arrest and apoptosis. Moreover, the mutant L1210 cell line was found to be more sensitive to the apoptotic effects of 2-HBA [1].
In vivo: The effect of 2-HBA on the DMBA-induced expression of the Ha-ras gene in isolated RNA tissues of CBA/Ca inbred mice was investigated. According to the previous findings, elevated Ha-ras expression was obserrved even 24 h after DMBA treatment. Administration of 2-HBA with DMBA could lead to a decrease of the DMBA-induced Ha-ras gene expression in all the investigated tissues, suggesting metabolic interaction of 2-HBA and DMBA. In addiiton, administration of 2-HBA 24 h prior to the DMBA treatment was able to reduce the Ha-ras gene expression in all tested tissues except the liver, which could be the result of a possible CYP1A inducer and pro-oxidant effects of 2-HBA [2].
Clinical trial: Up to now, 2-HBA is still in the preclinical development stage.
References:
[1] A. T. Dinkova-Kostova, A. H. Cory, R. E. Bozak, et al. Bis(2-hydroxybenzylidene)acetone, a potent inducer of the phase 2 response, causes apoptosis in mouse leukemia cells through a p53-independent, caspase-mediated pathway. Cancer Letters 245, 341-349 (2007).
[2] Perjési P, Ember I, Bozak RE, Nádasi E, Rozmer Z, Varjas T, Hicks RJ. Effect of the chalcone analog E,E-bis(2-hydroxybenzylidene) acetone on the 7,12-dimethylbenz[a]anthracene-induced Ha-ras gene activity in vivo. In Vivo. 2006 Jan-Feb;20(1):141-6.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 266.3 |
| Cas No. | 131359-24-5 |
| Formula | C17H14O3 |
| Synonyms | Bis(2-hydroxybenzylidene)acetone |
| Solubility | ≤14mg/ml in ethanol;11mg/ml in DMSO;12mg/ml in dimethyl formamide |
| Chemical Name | (1E,4E)-1,5-bis(2-hydroxyphenyl)-1,4-pentadien-3-one |
| SDF | Download SDF |
| Canonical SMILES | Oc1c(/C=C/C(/C=C/c(cccc2)c2O)=O)cccc1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure