ddCTP
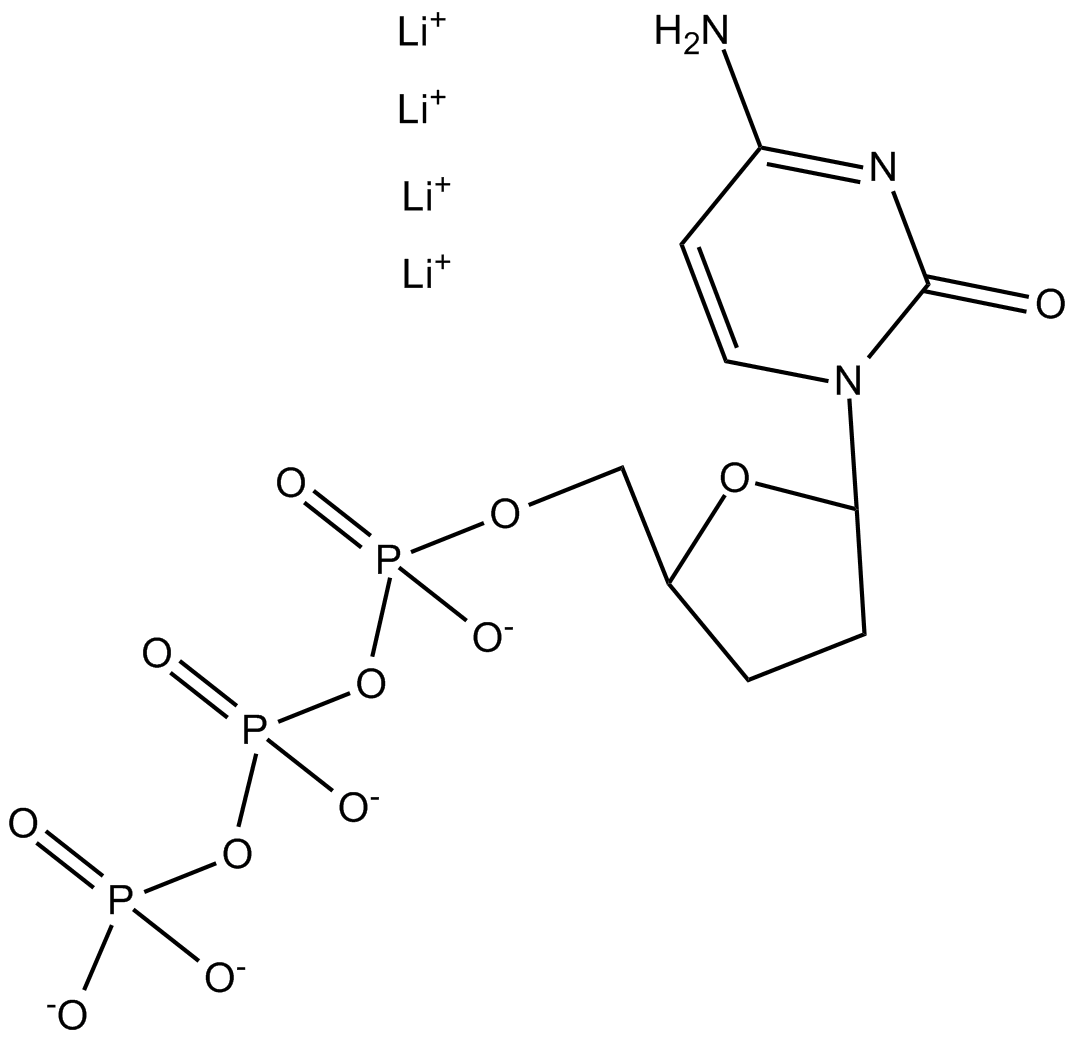
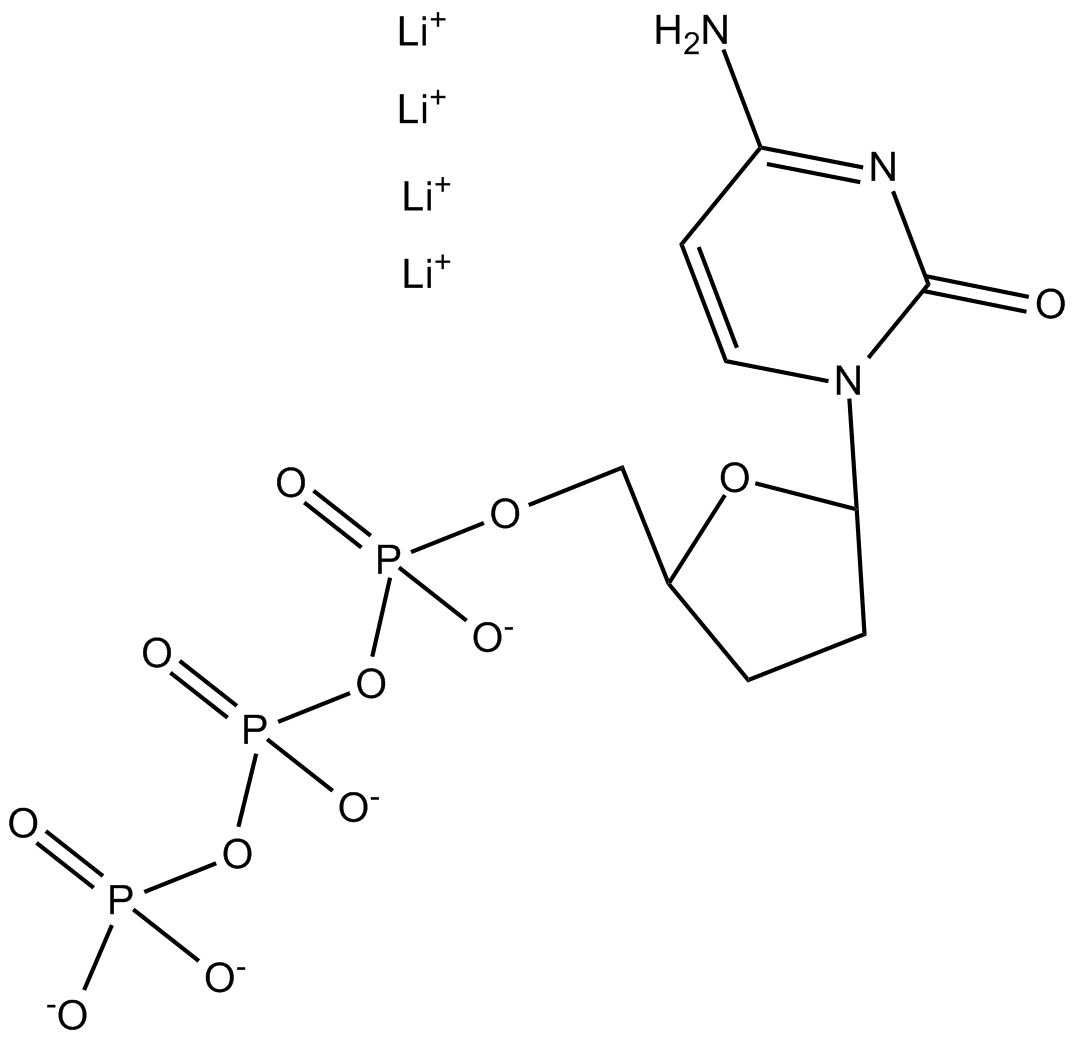
ddCTP (2',3'-Dideoxycytidine triphosphate) is a nucleotide analogue derived from cytidine, utilized primarily in nucleic acid biochemical research. Structurally lacking hydroxyl groups at both the 2' and 3' positions of the ribose sugar, ddCTP acts as a terminator substrate for DNA polymerases. Incorporation of ddCTP into a nascent DNA strand inhibits further elongation due to the absence of the essential 3'-hydroxyl group required for phosphodiester bond formation. Common experimental applications include DNA sequencing methodologies, primer extension assays, and polymerase activity investigations, allowing detailed analysis of nucleotide incorporation dynamics, DNA synthesis mechanisms, and polymerase substrate selectivity.
| Physical Appearance | Solution |
| Storage | Store at -20°C or below |
| M.Wt | 451.1 (free acid) |
| Formula | C9H16N3O12P3 (free acid) |
| Synonyms | 2',3'-Dideoxycytidine-5'-Triphosphate, Zalcitabine triphosphate |
| Chemical Name | lithium (5-(4-amino-2-oxopyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl triphosphate |
| SDF | Download SDF |
| Canonical SMILES | NC(C=CN1C2CCC(COP([O-])(OP([O-])(OP([O-])([O-])=O)=O)=O)O2)=NC1=O.[Li+].[Li+].[Li+].[Li+] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = ≥95% by AX-HPLC
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure